Translate this page into:
Vascular Endothelial Growth Factor, Pigment Epithelium-derived Factor, and Renal Function Determinants in Type 2 Diabetics in Kaduna, Northern Nigeria: A Case–Control Study
*Email: iyantui@yahoo.com
How to cite this article: Unung PJ, Bassey IE, Alhassan MB, Akwiwu EC, Effa FA, Etukudo MH, Udoh AE. Vascular Endothelial Growth Factor, Pigment Epithelium-derived Factor, and Renal Function Determinants in Type 2 Diabetics in Kaduna, Northern Nigeria: A Case Control Study. J Health Sci Res 2021;6(2):27-34.
Abstract
Context:
The role of vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF) on renal function of diabetics is unclear. Objective: This study investigated the relationship between VEGF, PEDF and urea, creatinine and estimated glomerular filtration rate (eGFR) in Type 2 diabetics.
Materials and Methods:
Hundred Type 2 diabetics and 50 controls were recruited into this case–control study. Analytes were determined by standard methods. Statistical significance was set at P < 0.05 and 95% Confidence interval.
Results:
VEGF, PEDF (P =0.0001), urea (P = 0.024), and creatinine (P = 0.026) were significantly higher in diabetics than controls but eGFR was comparable (P > 0.05) in both groups. VEGF and PEDF were comparable (P > 0.05) in the diabetics with eGFR <60 ml/min and >60 ml/min.
Conclusion:
plasma VEGF and PEDF are higher in diabetics but there seem to be no association between renal function determinants and either VEGF and PEDF.
Keywords
Creatinine
Estimated Glomerular Filtration
Pigment Epithelium-derived Factor
Type 2 Diabetes mellitus
Urea
Vascular Endothelial Growth Factor
Introduction
Diabetes mellitus is a global health problem that is among the five leading causes of death in most countries and places an enormous financial drain on the health-care sector as well as diabetic patients1. In 2019, the global diabetes prevalence was estimated to be about 9.3% which is equivalent to 463 million people having diabetes; this is projected to rise to about 10.9%, that is, 700 million persons with diabetes by 2045. Is has been estimated that in Africa, the prevalence of diabetes is 4.7%, that is, 19.4 million people with diabetes. This is also projected to rise to 5.2% (47.1 million people) in 20452. More than half of these people live in Sub-Saharan Africa, Nigeria inclusive1.
Diabetes mellitus predisposes patients to increased risk of complications such as diabetic nephropathy, neuropathy and retinopathy, cardiovascular disease, and dental disease3,4. Diabetic nephropathy is a complication of diabetes mellitus – occurring in 20–30% of Type 2 diabetics and accounts for significant morbidity and mortality because of the very high risk for chronic kidney disease (CKD). It occurs secondary to glycosylation, hypertension and renal hemodynamic changes, glycemic control, duration of disease, and genetic propensity5.
Nephropathy is heralded by a decline in the GFR which can be measured by creatinine clearance by direct or indirect methods such as estimated glomerular filtration (eGFR) and other renal function tests such as urea and creatinine6. The Cockcroft and Gault (CG) method of measuring eGFR is perhaps the most common practical approach among health-care professionals for estimating renal function after almost four decades of being in existence and despite inaccuracies that arise from variations in body composition among patients7. Although there are other methods such as the CKD epidemiology collaboration (CKD-EPI) formula and modification of diet in renal disease equation, they have been shown to be less accurate in Type 2 diabetics;8,9 hence, our choice of the CG equation in this study. National Kidney Foundation defines CKD as estimated GFR <60 mL/min by the CG formula10.
Vascular endothelial growth factor (VEGF) is an important chemokine that plays a significant role in angiogenesis, tissue growth as well as organ repair processes. Raised blood VEGF levels are thought to be involved in Type 2 diabetic complications, particularly diabetic retinopathy11. Much less is known about its role in diabetic nephropathy and results from studies about its role in diabetic nephropathy are controversial. Studies in animal models suggest that glomerular VEGF is a causal factor in diabetic nephropathy and its overexpression in podocytes is dangerous as it has been associated with massive proteinuria, advanced nodular glomerulosclerosis12. However, in animals and human experiments, reduction in glomerular VEGF expression was reported to generate glomerular filtration barrier lesions, proteinuria, and kidney failure13,14.
In spite of significant advances that have been made in the diagnosis and management of diabetes mellitus and complications associated with it, it remains a serious global health and socio-economic problem. It is, therefore, important to research on factors that affect the pathogenesis and progression of the complications in the diabetic patient population. This study assessed the levels of plasma VEGF, pigment epithelium-derived factor (PEDF), eGFR, serum urea, and creatinine in diabetics and determined the relationship between VEGF and PEDF and some renal function determinants in diabetics of a native black population.
Methods
This was a case–control study carried out in the National Eye Centre, Kaduna, Northern Nigeria from November 2017 to December 2018. The study enrolled 100 Type 2 diabetics of both genders and 50 apparently healthy controls recruited from around the Kaduna city. Their age range was 30–80 years. Simple random sampling technique was used. Diabetes was defined as diabetes as elevated fasting glucose >7.1 mmol/L on two occasions, the presence of Type 2 diabetes, and/or the use of antidiabetic medications. We also divided the diabetics into those eGFR<60 mL/min and those with GFR >60 mL/min as recommended by the National Kidney Foundation10. Before being enrolled in the study, written informed consent given by each subject and Ethical clearance was obtained from the National Eye Centre ethics and Research Committee (NECEC/ PATH/20170015). The tenets of the Declaration of Helsinki were strictly adhered to.
Sample size calculation
Power and sample size calculations for Unmatched Case-Control study were carried out using the StatCalc function of Epi-Info software (Centre for Disease control and Prevention). A two-sided confidence level of 95%, desired power of 80%, the ratio of controls to cases as 0.5, and odds ratio of 3.8 was utilized. The percentage outcome in the unexposed group was 11.4%15. The Fleiss formula16 gave a sample size of 90 for cases and 45 for controls. Calculating for an attrition rate of 10%, the sample size increased to 99 for cases and 50 for controls (i.e. a total of 149 participants).
Inclusion and exclusion criteria
Only type 2 diabetics of both genders were recruited as subjects. Type I diabetics and critically ill patients were excluded from the study.
Measurement of anthropometric indices and blood pressure and definition of cutoff
Blood pressure, weight (kg), and height (m) of each subject were measured as described elsewhere17. Body mass index (BMI) was given as a ratio of body weight (kg) and the square of body height (m2). Diabetes as elevated fasting glucose >7.1 mmol/L or the presence of Type 2 diabetes, and/or the use of antidiabetic medications18. Good control was defined as HbA1c ≤7% and poor glycemic control as HbA1c >7%19.
Sample collection and preparation
After an overnight fast, 6 ml of venous blood was aseptically obtained by venipunture at 8–10 AM from all participants. Two milliliters each of whole blood were dispensed into EDTA and sodium fluoride oxalate bottles for HbA1c estimation and plasma glucose estimation respectively. An aliquot of the EDTA sample was centrifuged for 15 min at 1000× g and the plasma obtained and stored at –20°C. The final 2 ml were used to obtain serum for urea and creatinine.
Laboratory assay methods
Blood glucose was estimated by a glucose oxidase colorimetric method obtained from Agappe reagent kits, China). HbA1c percentage was determined using a cation-exchange resin chromatography kit (Pointe Scientific Inc, USA). An ELISA kit obtained from PeproTech Inc. (Rocky Hill, USA) was used to determine plasma level of VEGF while an Aviscera ELISA kit from Bioscience (Santa Clara, USA) was used for assay of plasma level of PEDF. The procedures were carried out according to the manufacturer’s instructions. Estimated glomerular filtration rate (eGFR) was measured by the Cockcroft and Gault equation20.
Statistical analysis
Statistical Package for the Social Science version 18 (SPSS Inc. Chicago IL, USA) and R version 4.0.0 (R Foundation for Statistical Computing Platform, Vienna, Austria) were used to compute data obtained and results expressed as mean ± S.D. Relationship between categorical variables was tested using Chi-squared test of independence, normality of data by the Shapiro–Wilk test while Students’ t-test was used to compare continuous variables between groups. Correlations among parameters were evaluated by Pearson’s correlation coefficient (r). Statistical significance was set at P < 0.05 and 95% confidence interval.
Results
Demographic characteristics of the diabetics and controls are shown in Table 1. One hundred type 2 patients (51 males and 49 females) and 50 controls (24 males and 26 females) participated in the study. The mean duration of diabetes was 3.83 ± 4.22 years and about 77% of the diabetics had poor glycemic control.
| Variables | Diabetics | Controls | P-value |
|---|---|---|---|
| n=100 | n=50 | ||
| Age (years) | 57.0 (9.57) | 53.2 (15.48) | |
| n(SD) | |||
| Gender (n, %) | |||
| Female | 49 (49) | 26 (52) | 0.730 |
| Male | 51 (51) | 24 (48) | 0.730 |
| Hypertensiona (n, %) | 40 (40) | - | 0.0001* |
| Glycemic control (n, %) | |||
| Goodb | 23 (23) | - | |
| Poorc | 77 (77) | - | 0.0001* |
| Obesityd (n, %) | 30 (36) | 9 (18) | 0.024* |
| Duration of diabetes (year) | |||
| n(SD) | 3.83 (4.22) | - |
The body mass index, fasting plasma glucose, blood pressures, glycated Hb, VEGF, PEDF, urea, creatinine, and eGFR in patients with Type 2 diabetes mellitus and controls are shown in Table 2. Fasting plasma glucose, systolic BP, diastolic BP, HbA1c, VEGF, PEDF (P = 0.0001), urea (P = 0.024), and creatinine (P = 0.026) were significantly higher in diabetics than controls. However, there were no significant differences (P > 0.05) in the BMI and eGFR in both groups.
| Parameters | Diabetics n=50 | Controls n=50 | Calc. t | P-value |
|---|---|---|---|---|
| Body mass index (kg/m2) | 28.2±4.32 | 27.2±3.33 | 1.660 | 0.100 |
| Fasting plasma glucose (mmol/L) | 7.7±2.81 | 4.4±0.50 | 11.650 | 0.0001* |
| Systolic BP (mmHg) | 137.9±18.35 | 121.6±10.56 | 6.911 | 0.0001* |
| Diastolic BP (mmHg) | 86.2±12.73 | 78.2±7.46 | 4.796 | 0.0001* |
| HbAic (%) | 8.59±2.15 | 5.33±0.83 | 9.615 | 0.0001* |
| VEGF (µg/ml) | 40.9±11.96 | 31.7±6.84 | 5.988 | 0.0001* |
| PEDF (pg/mL) | 7.28±1.90 | 4.38±0.91 | 12.631 | 0.0001* |
| Urea (mmol/L) | 5.1±2.26 | 4.4±1.41 | 2.254 | 0.026* |
| Creatinine (µmol/L) | 78.8±33.32 | 69.1±18.45 | 2.277 | 0.024* |
| eGFR (ml/min) | 105.0±42.76 | 108.6±36.24 | 0.517 | 0.606 |
Results are expressed as mean±SD. *Significant at P<0.05. BP: Blood pressures, VEGF: Vascular endothelial growth factor, PEDF: Pigment epithelium-derived factor, eGFR: Estimated glomerular filtration rate
VEGF and PEDF levels in diabetics with diabetics with eGFR <60 ml/min and >60 ml/min are shown in Table 3. VEGF and PEDF levels were comparable in the diabetics with eGFR <60 ml/min and >60 ml/min (P > 0.05).
| Parameter | Diabetics with eGFR <60 ml/min (n=15) | Diabetics with eGFR ≥60 ml/min (n=85) | Calc. t | P-value |
|---|---|---|---|---|
| VEGF (µg/ml) | 38.5±12.27 | 41.3±11.93 | 0.843 | 0.401 |
| PEDF (pg/mL) | 7.17±1.98 | 7.30±1.90 | 0.249 | 0.804 |
Results are expressed as mean±SD. VEGF: Vascular endothelial growth factor, PEDF: Pigment epithelium-derived factor, eGFR: Estimated glomerular filtration rate
There were no correlations between any of the renal function parameters and either VEGF or PEDF. However, duration of disease correlated positively and significantly with VEGF (r = 0.203; P = 0.043; Figure 1) and PEDF (r = 0.276; P = 0.006; Figure 2).
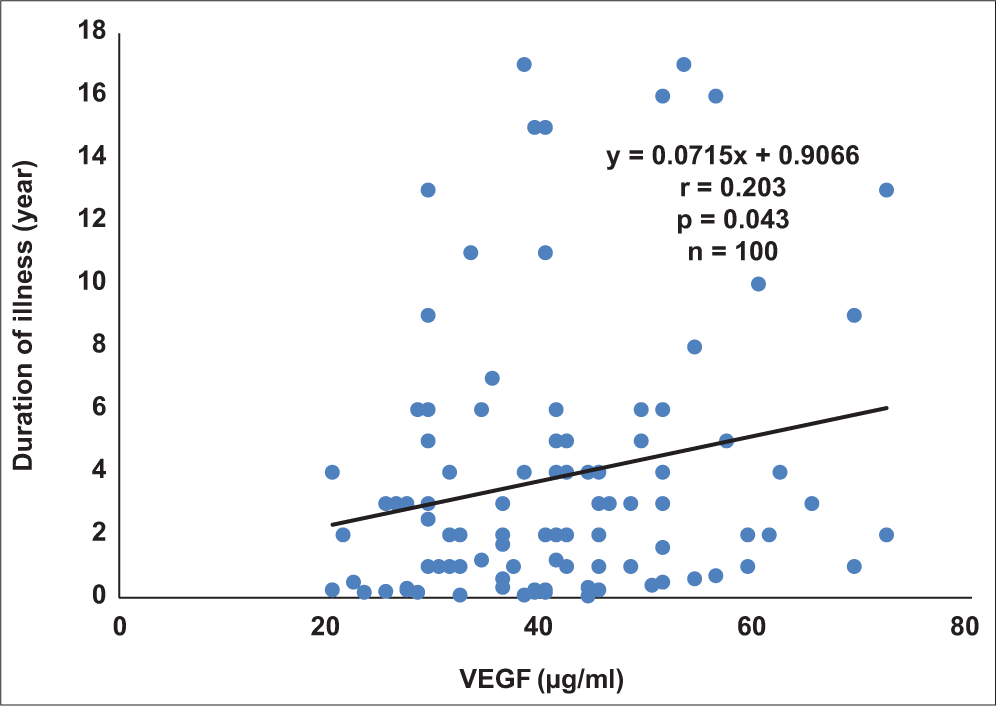
- Correlation plot of duration of illness against vascular endothelial growth factor in diabetics.

- Correlation plot of duration of illness against pigment epithelium-derived factor in diabetics.
Discussion
Diabetes mellitus is a chronic disease associated with complications like diabetic nephropathy which may result in ESRD increasing the morbidity and mortality of the disease21. Recent research is beginning to focus in the roles of biochemical factors such as VEGF and pigment epithelium derived factor (PEDF) in the development of these complications. Reports on the role of VEGF and PEDF in the development of diabetic nephropathy are controversial12-14.
It was observed that the frequency of hypertension among the diabetics was significantly higher compared to the controls and the frequency of diabetics with poor glycemic control was higher than those with good control. This is worrisome as one of the main ways of preventing and treating diabetic nephropathy is good glycemic control and blood pressure regulation3. Poor control of both of these variables i known triggers of increased VEGF production, which is one of the most potent proangiogenic factors and whose glomerular expression has been linked to diabetic nephropathy pathogenesis22.
The high frequency of poor glycemic control and hypertension observed in this study may be due to poor compliance to prescribed drugs or dietetic regiments which could be ascribed to several factors such as forgetfulness, poverty, perceived non-effectiveness, side effects, and pill burden23. It has been reported in a study by Abdulazeez et al. that more than 70% of the patients with diabetes did not comply with their prescribed drug regimen23. The prevalence of poor glycemic control reported in our study is higher than 62% and 46% reported in other parts of Nigeria24,25.
It was observed that VEGF levels were higher in the diabetics than the controls. Elevation of VEGF in vascular diseases may be caused by different mechanisms. First, a wide array of cytokines, growth factors and other molecules are released, in response to vascular damage. This stimulates angiogenesis through VEGF, which is crucial for the repair process26. Another probable mechanism is that elevation of VEGF may be a reflection of endothelial cell (EC) damage which occurs in diabetics27.
The high levels of VEGF in these subjects may be due to the over expression of VEGF gene in diabetic patients causing the induction of angiogenesis to compensate for tissue hypoxia. Hypoxia promotes the expression of homeostatic regulating genes, such as hypoxia inducible factor 1-α (HIF-1α) allowing tissues and cells to adapt to fluctuations in environmental conditions. HIF-1α regulates these responses to hypoxia by directing by mature ECs to migrate toward hypoxic environments through the HIF-1α regulation of VEGF transcription28,29. Our findings are in agreement with those of Mahdy et al.,30 who reported similar findings in diabetic patients.
The plasma PEDF levels, in this study, were also significantly higher in diabetic group compared to the controls. Increased VEGF – angiogenic stimulation triggers an increase in the production of PEDF it has a counter regulatory function and protects against hyperglycemia-induced damage to the vasculature caused by elevated VEGF levels31. PEDF has been reported to have anti-inflammatory, anti-oxidative, anti-thrombotic, and anti-angiogenic properties on vascular tissues32.
Therefore, its increase may help to combat the development of diabetes associated complications such as retinopathy and nephropathy and its therapeutic potentials are currently being explored33. The positive correlation observed between duration of disease with VEGF and PEDF probably explains why diabetes duration is independently associated with macro- and micro-vascular events34. This is because, increase in duration of disease coupled with poor glycemic control encourages the production of inflammatory mediators and proangiogenic factors such as VEGF which may give rise to complications in diabetics. This agrees with the findings of Mahdy et al.30.
Although urea and creatinine were higher in diabetics, the eGFR of both groups was comparable. Blood tests for urea and creatinine are the simplest way to monitor kidney function and are useful for the initial diagnosis of acute or CKD. Elevated serum urea and creatinine in the diabetics is an indication of declining kidney function35. However, the similarity in the eGFR of both groups may be an indicator to the fact that most of the diabetic group still had preserved kidney function as only 15 out of 100 of the diabetic patients in our study had eGFR <60 ml/ min. this agrees with Dabla,35 who reported that eGFR has limitations in patients with preserved kidney function.
Although both VEGF and PEDF were increased in the general diabetic group, the levels of both markers in diabetics with eGFR <60 ml/min and >60 ml/min were not statistically different and there were no correlations between any of the renal function parameters with either VEGF or PEDF. This is contrary to the findings in a study by Zhao et al.,36 who reported higher VEGF levels in diabetics with nephropathy.
Contrary to our findings also Shiga et al.37 observed that plasma PEDF levels are significantly increased in patients with CKD than patients without CKD and Matsuyama et al.38 reported higher levels of PEDF in Type 2 diabetic patients with nephropathy. The reason for this discrepancy is not clear. Some studies have, however, shown that decreased PEDF levels in the kidney are implicated in diabetic nephropathy (Wang et al., 2005). The increased PEDF levels observed in the diabetic group in this study may have helped in preserving kidney function resulting in the comparable eGFR observed in both groups.
Experimental animal models have shown that administering an adenovirus expressing the PEDF gene39 and P78-PEDF – small bioactive peptide fragment of PEDF40 reduced albuminuria significantly, ameliorated glomerular hypertrophy, and inhibited fibronectin expression in the kidney39. PEDF blocks the survival, proliferation, and migration of ECs by several mechanisms including attenuating β-catenin nuclear translocation and the consequent expression of angiogenic genes such as VEGF; competing with VEGF for binding to KDR; inhibition of the angiogenic NFAT/c-FLIP and Akt/MAPK pathways; suppressing the survival signaling of EC by stimulating γ-secretase to cleave Flt-1; inhibition of ATP production needed for EC angiogenic events by binding to the F1-ATP synthase β-subunit; and promotion of the apoptosis of ECs through the activation of JNKs,p38, PPAR-γ, and NF-κ41.
In addition, PEDF has been shown to inhibit hyperglycemia-induced activation of NF-B, a crucial transcription factor that mediates inflammatory responses, and HIF-1α, which plays a key role in the activation of the expression VEGF. This suggests that protective effect of PEDF against diabetic nephropathy may be somewhat through its anti-inflammatory activity33.
In this study, we observed that both VEGF and PEDF are increased in type 2 diabetics and their levels increase with increase in duration of disease. The urea and creatinine were higher in diabetics but eGFR was comparable between diabetics and controls. VEGF and PEDF levels were not significantly different in diabetic patients with eGFR <60 ml/min and >60 ml/min and there were no significant correlations between either VEGF and PEDF and the renal function determinants.
A limitation of this study was that microalbumin test was not carried out to detect those with early diabetic nephropathy as that might have helped shed more light on the pattern of results obtained in this study.
Acknowledgment
We would like to thank the doctors and patients of the National Eye Hospital for participating in this study.
Conclusion
Plasma VEGF and PEDF are higher in diabetics but there seem to be no association between renal function determinant and either VEGF and PEDF.
Authors’ Contribution
Unung PJ, Udoh AE, and Etukudo MH research conceptualization and design, Bassey IE, Unung PJ and Udoh AE analyzed and interpreted the data. Bassey IE, Unung PJ, and Effa FA performed the experiment and writing the initial draft manuscript while Esiere KUS, Akwiwu EC critically reviewed the manuscript.
Conflict of interest
The authors have no conflict of interest to declare
Data Availability Statement
Data will be available from authors on reasonable request.
Funding
Nil.
References
- Cost of diabetes mellitus in Africa: A systematic review of existing literature. Global Health. 2018;14(1):3.
- [CrossRef] [PubMed] [Google Scholar]
- Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition In: Diabetes Res Clin Pract. Vol 157. 2019. p. :107843.
- [CrossRef] [PubMed] [Google Scholar]
- The pattern of dietary habits and glycemic control of diabetics in eastern Nigeria. Pak J Nutr. 2006;5(1):43-45.
- [Google Scholar]
- Evaluating all potential oral complications of diabetes mellitus. Front Endocrinol (Lausanne). 2019;10:56.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic nephropathy complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361-81.
- [PubMed] [Google Scholar]
- Cockcroft-Gault revisited: New de-liver-ance on recommendations for use in cirrhosis. World J Hepatol. 2017;9(3):131-8.
- [CrossRef] [PubMed] [Google Scholar]
- The chronic kidney disease epidemiology collaboration (CKD-EPI) equation is less accurate in patients with Type 2 diabetes when compared with healthy individuals. Diabet Med. 2011;28(1):90-5.
- [CrossRef] [PubMed] [Google Scholar]
- The chronic kidney disease epidemiology collaboration (CKD-EPI) formula performs worse than the modification of diet in renal disease (MDRD) equation in estimating glomerular filtration rate in Type 2 diabetic chronic kidney disease. Diabet Med. 2011;28(10):1279.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health. 2008;8:117.
- [CrossRef] [PubMed] [Google Scholar]
- What is the contribution of two genetic variants regulating VEGF levels to Type 2 diabetes risk and to microvascular complications? PLoS One. 2013;8(2):e55921.
- [CrossRef] [PubMed] [Google Scholar]
- Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of Type 1 diabetes. Diabetologia. 2011;54(5):1227-41.
- [CrossRef] [PubMed] [Google Scholar]
- Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61(11):2958-66.
- [CrossRef] [PubMed] [Google Scholar]
- Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts alphaVbeta3 integrin signaling in the glomerulus. PLoS One. 2012;7(7):e40589.
- [CrossRef] [PubMed] [Google Scholar]
- Towards prevention of chronic kidney disease in Nigeria: A community-based study in Southeast Nigeria. Kidney Int Suppl. 2013;3(2):195-201.
- [CrossRef] [Google Scholar]
- Statistical Methods for Rates and Proportions (2nd ed). New York: John Wiley; 1981.
- [Google Scholar]
- Impact of androgen deprivation on oxidative stress and antioxidant status in Nigerian patients with prostate cancer undergoing androgen deprivation therapy. JCO Glob Oncol. 2020;6:1481-9.
- [CrossRef] [PubMed] [Google Scholar]
- Standards of medical care in diabetes-2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14-37.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
- [CrossRef] [PubMed] [Google Scholar]
- Antiangiogenic therapy for diabetic nephropathy. Biomed Res Int. 2017;2017:5724069.
- [CrossRef] [Google Scholar]
- Medication adherence amongst diabetic patients in a tertiary healthcare institution in Central Nigeria. Trop J Pharm Res. 2014;13(6):997-1001.
- [CrossRef] [Google Scholar]
- Glycaemic control amongst persons with diabetes mellitus in Benin City. Niger Med J. 2010;51:164-6.
- [CrossRef] [Google Scholar]
- Glycaemic control amongst diabetic mellitus patients in Umuahia Metroppolis, Abia State, Nigeria. Int J Basic Appl Inn Res. 2012;1:98-104.
- [Google Scholar]
- Molecular mediators of angiogenesis. J Burn Care Res. 2010;31(1):158-75.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension. Clin Sci (Lond). 2005;109(1):39-43.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80(2):51-60.
- [PubMed] [Google Scholar]
- Effect of glycemic control and dyslipidemia on plasma vascular endothelial growth factor and pigment epithelium-derived factor in diabetic retinopathy patients in Northern Nigeria. Int J Health Sci (Qassim). 2020;14(6):4-12.
- [PubMed] [Google Scholar]
- The role of vascular endothelial growth factor in the progression of diabetic vascular complications. Eye (Lond). 2010;24(10):1576-84.
- [CrossRef] [PubMed] [Google Scholar]
- Genotypes and phenotypes: A search for influential genes in diabetic retinopathy. Int J Mol Sci. 2020;21(8):2712.
- [CrossRef] [PubMed] [Google Scholar]
- Serum pigment epithelium-derived factor levels are independently correlated with the presence of coronary artery disease. Cardiovasc Diabetol. 2013;12:56.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F1166-73.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in Type 2 diabetes. Diabetologia. 2014;57(12):2465-74.
- [CrossRef] [PubMed] [Google Scholar]
- Renal function in diabetic nephropathy. World J Diabetes. 2010;1(2):48-56.
- [PubMed] [Google Scholar]
- Relationship of serum 8-OHdG and VEGF with diabetic nephropathy in diabetics. Chin J Diabetes. 2012;20(9):667-70.
- [Google Scholar]
- Significance of plasma levels of pigment epithelium-derived factor as determined by multidetector row computed tomography in patients with mild chronic kidney disease and/or coronary artery disease. J Int Med Res. 2011;39(3):880-90.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between pigment epithelium-derived factor (PEDF) and renal function in patients with diabetic retinopathy. Mol Vis. 2008;14:992-6.
- [PubMed] [Google Scholar]
- Decreased expression of pigment epithelium-derived factor is involved in the pathogenesis of diabetic nephropathy. Diabetes. 2005;54(1):243-50.
- [CrossRef] [PubMed] [Google Scholar]
- Protective role of small pigment epithelium-derived factor (PEDF) peptide in diabetic renal injury. Am J Physiol Renal Physiol. 2013;305(6):F891-900.
- [CrossRef] [PubMed] [Google Scholar]
- PEDF and its roles in physiological and pathological conditions: Implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond). 2015;128(11):805-23.
- [CrossRef] [PubMed] [Google Scholar]






