Translate this page into:
COVID-19 infection and exercise reveal the ECG form of Brugada syndrome: A two-case reports
*Corresponding author: Dr. Ioannis Vogiatzis, Department of Cardiology, General Hospital of Veroia, Asomata, Veroia, Greece. ivogia@hotmail.gr
-
Received: ,
Accepted: ,
How to cite this article: Sdogkos E, Konstantinou T, Spahiu A, Vogiatzis I. COVID-19 infection and exercise reveal the ECG form of Brugada syndrome: A two-case reports. J Health Sci Res. 2025;10:25-31. doi: 10.25259/JHSR_61_2024
Abstract
The specific electrocardiogram (ECG) form of Brugada syndrome (BrS) in V1-V3 leads is not always evident and is revealed after the impact of exogenous factors. In the first case, a 57-year-old man is described who came to the hospital with COVID-19 infection and febrile. During his hospitalization, a pattern of Brugada syndrome (BrS) type I appeared on the ECG, which persisted as long as the febrile lasted and disappeared with the retreat of the febrile. The patient refused further investigation. Family history was negative for sudden death. The second case describes a 55-year-old man who underwent an exercise test for preventive reasons. In the five minutes of exercise, the BrS pattern appeared on ECG without further symptomatology. He remained on the recovery ECG thereafter for 15 minutes after the end of the exercise. He underwent an electrophysiological study (ventricular stimulation) which induced nonsustained ventricular tachycardia with isoproterenol administration. No history of sudden death is reported in his family. Fever and exercise are two important factors in revealing the ECG pattern of BrS in patients with this canalopathy.
Keywords
Brugada syndrome
Coronavirus
COVID-19
Exercise test
Fever
INTRODUCTION
Brugada syndrome (BrS), which primarily affects men and is characterized by abrupt, unexplained cardiac mortality in healthy persons, was initially reported in the 1990s.[1]
The electrocardiogram (ECG) is characterized by ST elevation accompanied by negative T in the right precordial leads, with no structural cardiac abnormality and pseudo-right bundle branch block (RBBB).[2] Later descriptions of mutations in the SCN5A and SCN10A genes, which affect Na+ channels in the cardiac tissue, explained the origin of around 50% of cases.[3] Genetic studies have shown that mutations in SCN5A and SCN10A genes are associated with multiple cardiac diseases, like BrS, long QT syndrome, conduction diseases, and cardiomyopathy, while genetic variation in the general population has been associated with the risk of arrhythmia.
The phenotype of BrS is associated with a decrease in Na+ current due to the elimination of Na+ channels and/or a decrease in Na+ channel functionality. Patients with this condition are frequently healthy and unaware of this hereditary predisposition, despite the presence of diagnostic ECG abnormalities. The condition is identified during a syncopic episode or an aborted sudden death.[4]
The triggering elements that lead to arrhythmia in people with the condition have been identified as being fever, alcohol, parasympathetic tone augmentation, and medicines that block Na+ channels.[5]
As a result, several infectious and noninfectious illnesses that induce fever unexpectedly reveal the BrS pattern on the ECG. There have been cases recently where the BrS ECG pattern was observed by fever from the COVID-19 disease.[6]
Another uncommon case is the occurrence of BrS type I in exercise. It is uncommon in clinical practice, and isoproterenol is the treatment if an electrical storm occurs.[7]
We describe two cases where the ECG pattern of BrS appeared after a febrile due to the COVID-19 disease and in the second case after a stress test performed for preventive reasons.
CASES SERIES
Case 1: A 57-year-old man arrives at the Emergency Department of our hospital with a fever of 39°C and chills that initiated 36 hours before his arrival. The ECG is shown in Figure 1a.
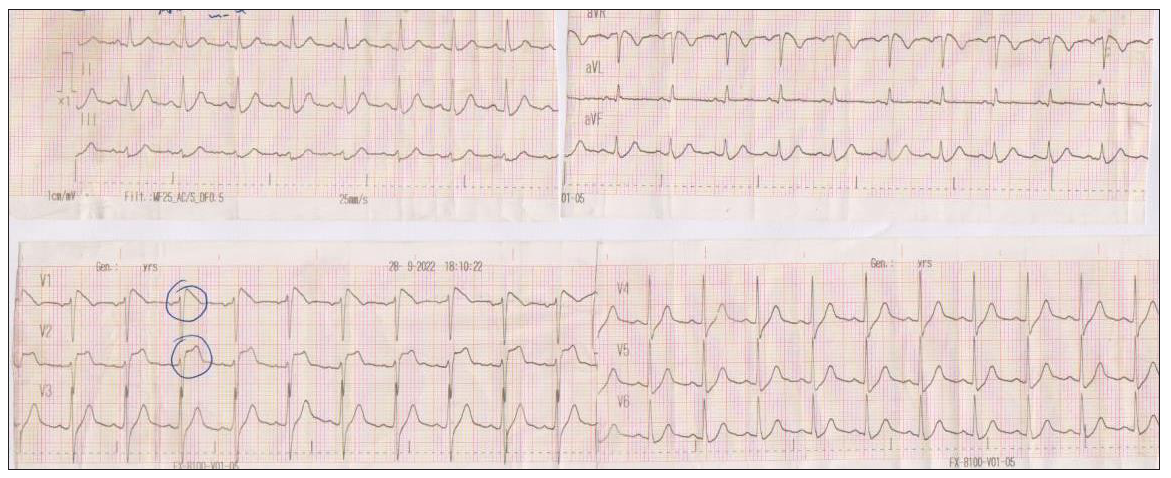
- The initial ECG of the patient in the Emergency Department with a fever of 39°C. ECG: Electrocardiogram
He was initially tested for COVID-19 via a rapid test and was positive. He was subsequently admitted to the hospital for further investigation. The fever was controlled with antipyretics and remained asymptomatic without respiratory system symptoms (cough, dyspnea). No arrhythmias such as premature ventricular contractions and/or ventricular tachycardia (VT) (sustained or not sustained) were observed. The patient was afebrile 48 hours after the admission, and the ECG was altered [Figure 1b] remaining for the duration of the patient’s hospitalization. After six days of hospitalization, the rapid test was negative and the patient was discharged from the hospital.
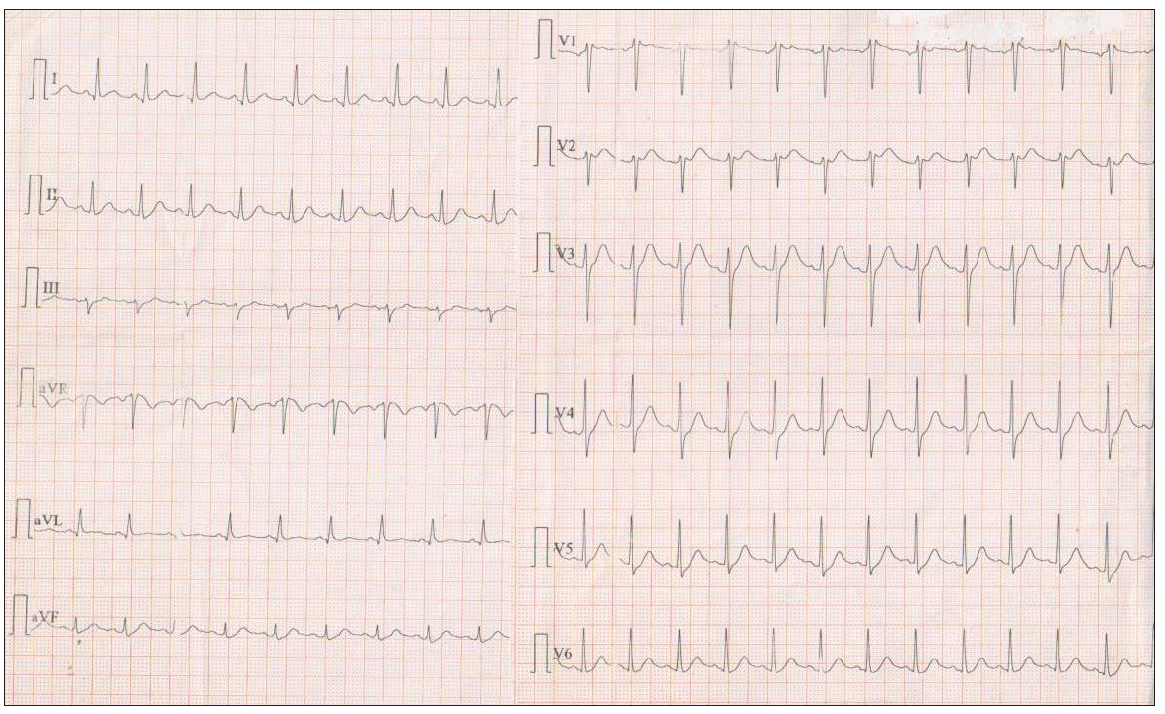
- The ECG of the patient after the resolution of the fever. ECG: Electrocardiogram
There was no family history of sudden cardiac death (SCD), even though the brother reported a syncopal episode a year ago. Whether the brother’s syncopal episode was associated with the possible presence of BrS in the members of this family remains unknown.
He was recommended further testing for the COVID-19 and for BrS pattern on the ECG. The patient did not attend the scheduled appointment.
Case 2: A 55-year-old man came to the Prevention Clinic for a checkup. Due to the presence of risk factors for atherosclerotic disease (hypertension, diabetes, dyslipidemia), he was subjected to an exercise test. He was not taking any medication. Family history was negative for coronary heart disease or SCD. The ECG was normal with a saddle-like ST segment elevation in V1-V2 leads consistent with BrS type 2 pattern [Figure 2a] and the echocardiogram (ECHO) showed normal cavity dimensions, normal valves, and preserved ejection fraction (EF).

- Baseline ECG at the initial visit. ECG: Electrocardiogram
Gradual dynamic ECG changes appeared after 2:50 minutes of exercise at 136 bpm [Figure 2b, 2c]. ECG changes became more pronounced at peak exercise at 8΄25˝ and heart rate at 183 beats/min. The ST intervals in the V1-V2 leads in the fourth intercostal interval became more pronounced and turned to a coved pattern, same as the pattern of type 1 BrS. In recovery, the ST intervals gradually returned to baseline at the tenth minute.

- Stress test. Initial ECG with a saddle-like ST segment elevation in V1-V2 leads consistent with Brugada syndrome type 2 pattern. ECG: Electrocardiogram
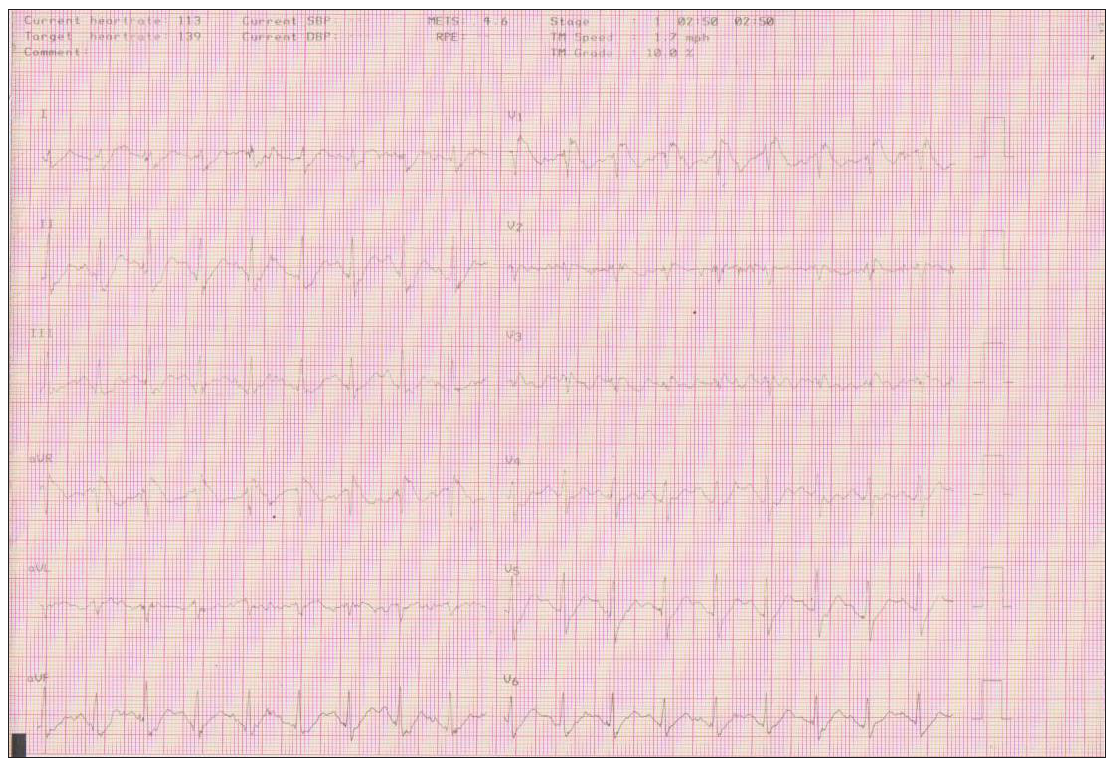
- Stage 1 of the Bruce protocol where the ECG Brugada pattern appears at 2΄ 50΄΄ duration of exercise and heart rate at 136 beats/min. ECG: Electrocardiogram
The patient achieved the maximum heart rate (162 beats) of the Bruce protocol without developing symptomatology or arrhythmias during exercise and recovery [Figure 2d].
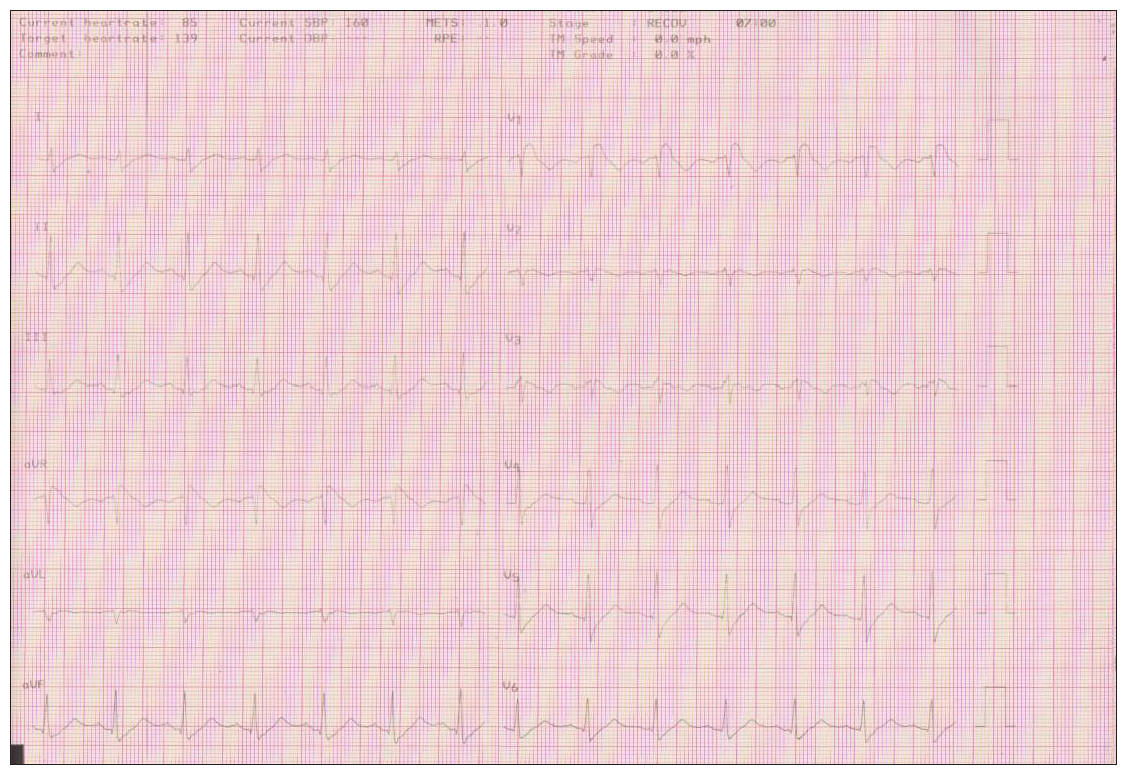
- It remained the entire recovery period till the tenth minute.
A pharmacological provocative test by the administration of procainamide was performed and the BrS type I was presented on the ECG.
The patient underwent an electrophysiological study. At the essential stage of the study, BrS pattern was not apparent on the ECG. Conduction intervals were within the normal range (HV = 45 msec). Nonsustained VT was induced during programmed electrical stimulation protocol at 500 msec cycle length and two extrastimulus 200/340 msec after isoproterenol administration [Figure 2e].
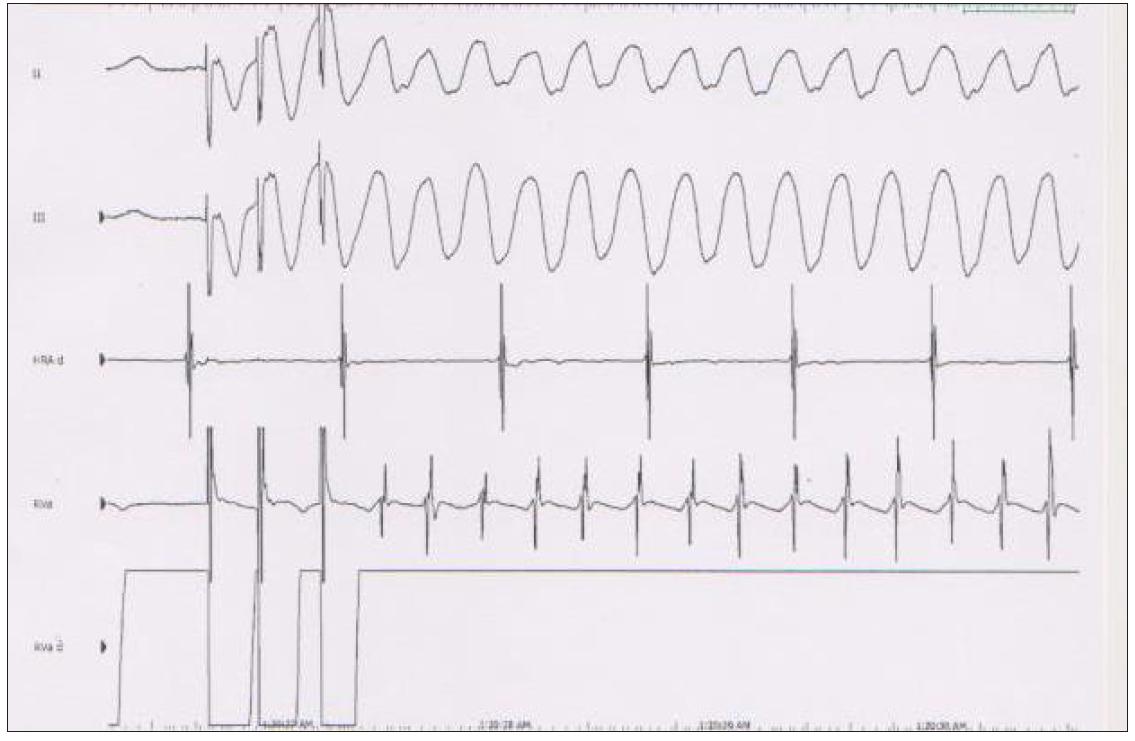
- Nonsustained ventricular tachycardia (VT) was induced during programmed electrical stimulation protocol at 500 msec cycle length and two extrastimulus 200/340 msec after isoproterenol administration.
After this, a beta blocker was administered, and it was decided after discussion with the patient not to implant cardioverter-defibrillator (ICD). During a six-month follow-up, the patient reported no problems.
The first-degree relatives were examined and they had a normal ECG and the exercise tests were normal without the BrS pattern provocation.
DISCUSSION
The two most prevalent BrS ECG forms are type I with ST elevation and the convex upward and reversed T waves in leads V1-V2, and Type II with saddleback ST-segment elevation and biphasic T waves in leads V1-V2.[7] The risk of arrhythmias is higher in type I.[8]
Patients who exhibit the BrS Type I pattern and have clinical characteristics such as a history of syncope, ventricular arrhythmia, and a family history of sudden death are strongly recommended to undergo an ICD implantation.[9]
Although the arrhythmias during sleep are usually benign, nevertheless we must not ignore the fact that circadian rhythm disorders can affect the function of the autonomic nervous system, and therefore their presence, and make them potentially dangerous, especially with the existence of structural or ischemic heart disease. Ventricular arrhythmias in BrS commonly occur during nocturnal periods or when the individual is asleep, regardless of whether they are at rest or experiencing bradycardia. Moreover, cholinergic activity is crucial for arrhythmiogenesis in Br S. Furthermore, the level of activity in the autonomic nervous system, namely the parasympathetic activity, has an impact on the extent of ST abnormalities.[10,11] Conversely, a resolution of ST anomalies has been observed in sympathetic activity.[12] This could explain the administration of isoproterenol in BrS with VT storm and the avoidance of beta blockers.
The BrS type I pattern in the exercise test appears in recovery, as one recent review has shown. Out of the 166 individuals who conducted an exercise test, only two showed ST elevation during exercise. This demonstrates that exercise-induced sympathetic activation leading to an increase in HR can trigger a more malignant type (mutation?) BrS pattern type I.[13]
In addition, a mutation is described that exhibits ST segment augmentation at higher cardiac rates affects the C-terminal portion of Na channels that increase the slow inactivation and decelerate the recovery of Na+ channels availability. This explains the reason the exercise reveals the BrS pattern type I in this subgroup of patients. It is worth noting that people with this mutation experience QT prolongation at lower cardiac rates.[14]
Exercise intensity and heart rate at the peak of exercise contribute significantly to the appearance of BRS type I. In our case, the HR in the exercise rate reached up to 150 with the appearance of the pattern earlier. Exercise intensity and HR in peak exercise contribute significantly to the occurrence of BRS type I.
A genetic test was not performed on the patient. An electrophysiological study was conducted in which a nonsustained VT was induced following the administration of isoproterenol.
The uncommon response to the exercise observed in our case is attributed to a specific mutation that interferes with the time-dependent properties of Na+ channels. In recovery, fewer Na+ channels are observed, resulting in a lower conduction rate and a relative increase in forces leading to early repolarization. The modifications to conduction and repolarization appear to be explained by this theory.[15]
The patient of the case underwent an exercise test for preventive reasons. However, the BrS pattern appeared unexpectedly in exercise without the occurrence of VT. It was induced, although not sustained in the electrophysiological study, by the administration of isoproterenol (a b-receptor agonist). Although limited data exist on the utilization of the exercise test to diagnose BrS, our case implies that it could be a useful tool in stratifying risk to exercise for these patients. In contrast, exercise test is not considered a routine test for risk stratification in these patients. The role of the exercise test in the diagnosis and stratification of risk in first-degree relatives without prior ECG pattern BrS type I has also not been elucidated. Discussion is generated about the predictive value of ventricular arrhythmia[16] produced during the electrophysiological study—ventricular stimulation—in the laboratory in asymptomatic patients with BrS.
Experts recommend that ICD may be considered in patients with BrS who experience arrhythmia in electrical stimulation. The data are conflicting.[17,18] However, most scientists insist on negative predictive value. Generally, asymptomatic patients with ECG Brugada pattern with no clinical signs and family history are not favored for ICD implantation.[19]
Factors that trigger the syndrome, such as fever, alcohol, and drugs, reveal the ECG BrS pattern. According to studies, at least half of these patients experienced syncope or SCD while they were feverish.[20,21] Sodium channels’ biophysical properties decline when exposed to high temperatures. This deterioration is minimal; however, it predisposes to arrhythmias.[22] In addition, shortening of the dispersion of the duration of the action potential by increased temperature may facilitate VT due to reentry.[23]
In clinical practice, hyperthermia might convert a hidden BrS (with ECG pattern type 2 or 3 or more infrequently with normal ECG) into pattern type I. Another undesired aspect that should be avoided in individuals with known BrS, whether obvious or hidden, is hypothermia.[24] The morphology of BrS occurs in the ECG of a patient with fever of any etiology, mainly in respiratory infection. In a recent review, the rate was 83%.[25]
COVID-19 was described in 2019 in China as a case of pneumonia of unknown etiology. In January 2020, the new virus was identified and the rapid spread was declared a pandemic in March 2020.[20] Severe inflammatory reaction leads to febrile disease in the vast majority of patients. In our case, the infection led to a febrile status and in the BrS pattern on the ECG. Fever seems to trigger the arrhythmia in patients with BrS. Studies showed that at least half of patients suffered syncope or sudden death in febrile conditions.[20,22]
The reason why the description of the specific cases was interesting has to do with raising the awareness of the attention of healthcare providers in cases where the specific BrS can potentially be revealed, especially by doctors other than cardiologists, in order for these patients to carry out the necessary diagnostic workup.
CONCLUSION
Fever and exercise are two important factors in revealing the ECG pattern of BrS in patients with this canalopathy. The careful study of the electrocardiogram is significant in order not to miss cases of asymptomatic for the specific syndrome individuals who may have the specific variant in order to perform the required risk stratification control to them or to their relatives.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Sudden unexplained death syndrome in Southeast Asia. Am J Cardiol. 1997;79:10-1.
- [CrossRef] [PubMed] [Google Scholar]
- The syndrome of right bundle branch block ST segment elevation in V1 to V3 and sudden death—the Brugada syndrome. Europace. 1999;1:156-66.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol. 2014;64:66-79.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Brugada syndrome: A general cardiologist’s perspective. Eur J Intern Med. 2017;44:19-27.
- [CrossRef] [PubMed] [Google Scholar]
- Brugada syndrome: Clinical, genetic, molecular, cellular, and ionic aspects. Curr Probl Cardiol. 2016;41:7-57.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pyrexia unmasking Brugada syndrome: A literature review. Cureus. 2022;14:e22489.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol. 2012;45:433-42.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm. 2016;13:1274-82.
- [CrossRef] [PubMed] [Google Scholar]
- 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997-4126.
- [CrossRef] [PubMed] [Google Scholar]
- ST-segment elevation and ventricular fibrillation without coronary spasm by intracoronary injection of acetylcholine and/or ergonovine maleate in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1841-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:667-73.
- [CrossRef] [PubMed] [Google Scholar]
- Successful prevention of recurrent ventricular fibrillation by intravenous isoproterenol in a patient with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1293-4.
- [CrossRef] [PubMed] [Google Scholar]
- Brugada syndrome, exercise, and exercise testing. Clin Cardiol. 2015;38:323-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Two distinct congenital arrhythmias evoked by a multidysfunctional Na(+) channel. Circ Res. 2000;86:E91-7.
- [CrossRef] [PubMed] [Google Scholar]
- Exercise-induced Brugada syndrome type 1 pattern. HeartRhythm Case Rep. 2022;8:288-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Exercise-induced syncope and Brugada syndrome. Ann Pediatr Cardiol. 2019;12:292-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk stratification in Brugada syndrome: Results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37-45.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada syndrome registry. Circulation. 2010;121:635-43.
- [CrossRef] [PubMed] [Google Scholar]
- Risk stratification of the patients with Brugada type electrocardiogram: A community-based prospective study. Europace. 2009;11:507-13.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 Infection unmasking Brugada syndrome. HeartRhythm Case Rep. 2020;6:237-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149:216-8.
- [CrossRef] [PubMed] [Google Scholar]
- Brugada syndrome and fever: Genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res. 2005;67:510-9.
- [CrossRef] [PubMed] [Google Scholar]
- Induced Brugada-type electrocardiogram, a sign for imminent malignant arrhythmias. Circulation. 2008;117:1890-3.
- [CrossRef] [PubMed] [Google Scholar]
- Fever-induced type-1 Brugada pattern: A sign of revealed Brugada syndrome or just a Brugada phenocopy? Kardiol Pol. 2022;80:1161-2.
- [CrossRef] [Google Scholar]
- Brugada syndrome unmasked by fever: A comprehensive review of literature. J Community Hosp Intern Med Perspect. 2020;10:224-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







