Translate this page into:
Anthropogenic Activity and Aquatic Health with References to Hepatotoxicity and Endocrine Disruption in Chrysichyths furcatus
*Email: ikpesuto@fuotuoke.edu.ng
How to cite this article: Ohwofasa IT, German IC. Anthropogenic Activity and Aquatic Health with References to Hepatotoxicity and Endocrine Disruption in Chrysichyths furcatus. J Health Sci Res 2023;8(1):11-18.
Abstract
Anthropogenic activities had affected aquatic life in the nascent Niger-Delta ecological zone; hence, the hepatotoxicity and endocrine disruption in one of the dominant freshwater fish in the region were investigated. Post juvenile Chrysichthys furcatus of relatively equal sizes and weight 15.0 ± 0.30 g and 11.40 ± 0.1 cm, respectively, were chosen for this investigation. The fish that were purchased from a private fish farm, were acclimatized for 14 days and exposed to the spectrum of values measured in the wild (250, 500, and 750) mg/100 mL and a control. The water in the aquaria was kept within the standard limit with the aid of aerators. Blood chemistry was determined using standard methods. Physiological responses of the fish exposed to the crude oil were designated based on the severity, good condition, and abnormality. The hepatic response indices were affected, and the endocrine disruption marker decreased with an increase in the toxicant concentrations. These findings had revealed that crude oil is detrimental to fish health, and there is a tendency of affecting biodiversity and magnification along with the trophic level. Its, therefore, becomes imperative that appropriate authorities should take the necessary step to avoid the spillage of oil into the aquatic environment
Keywords
Anthropogenic activities
Chrysichyths furcatus
Crude oil
Endocrine disruption
Hepatotoxicity
Introduction
The Niger Delta is critical to the country’s oil industry. However, gas flaring and oil spills have had the greatest impact on this area, which spans over 70,000 km2 and contains 800 oil-producing communities. The Niger Delta has become a byword for oil pollution due to the region’s prevalence of oil production facilities (Over 900 oil wells, 100 flow stations/gas plants, 1500 km of trunk lines, and 45,000 km of oil/gas flow lines are in the region)1.
Each stage of oil and gas extraction poses risks to the expanding biological zone and its inhabitants2. Surveying, cleaning seismic lines, and huge dynamiting for geological excavations are all part of seismic companies’ oil exploration processes. Seismic businesses use surveying equipment and explosives to open paths for seismic lines and create large holes in the ground to excavate for oil. Exploding dynamite in water causes a toxic environment that kills fish and other aquatic creatures3. Dynamite firing destabilizes sedimentary materials, which increases turbidity, hinders the filter- feeding mechanisms of benthic (bottom-dwelling) creatures, and lowers plant photosynthetic activity due to reduced light penetration.
Not only does cleaning pipeline lines reduce habitat area but it also physically separates natural populations, which could alter mating patterns. The Niger Delta is a frequent location for oil spills, and various activities contribute to oil’s introduction into the environment, such as pipeline leaks and ruptures, unintentional discharges (such as tank accidents), and discharges from refineries and urban centers.
Environmental contamination produced by oil prospecting and exploration activities has negatively impacted the biodiversity of the Niger Delta region of Nigeria. Changes in the freshwater fauna and flora of this region of Nigeria have been observed. To protect the environment from oil exploration, the government has adopted rules, but these must be adequately implemented, enforced, and monitored by the appropriate agencies. Thus, this study aims to evaluate the effects of crude oil in the wild by subjecting a prominent fish in the area to quantities found during field investigations and then measuring endocrine and oxidative stress enzymes as biomarkers.
Materials and Methods
Study area
The experiment was conducted from May through December 2021. Raw unadulterated crude oil (98 octanes) was obtained from a Chevron company, Warri Nigeria. The water-soluble fraction (WSF) was obtained as described by Igbiri et al.4. One-part crude oil was added to ten parts water in a glass container and stirred vigorously for 20 h at room temperature. The mixture was allowed to settle for a while to separate the water from the insoluble components. Following the removal of insoluble components, the aqueous phase was collected and diluted to field concentrations (250, 500, and 750 mg/L) encountered by aquatic species.
Experimental fish and laboratory setup
Juvenile fish of similar size and weight (15.0 0.30 g and body length of 11.40 0.1 cm) were chosen for this study, because they provide a low-cost model for testing potential endocrine disruption chemicals (EDCs), with the added benefit of allowing for the integration of molecular to phenotypic and functional effects.
The fish were bought from a private fish farm in Delta State and transported first thing in the morning to lessen the impact of the heat stress that would have occurred at any other time of day. In the laboratory, they were put into big capacity water tanks and supplied for a minimum duration of 14 days to acclimatize to the laboratory condition. During this period, they were fed twice daily with rice bran and groundnut oil cake in a dough form. Excess amounts of feed and fecal wastes were removed daily to avoid contamination of water, and water was refilled twice weekly.
Toxicity test
After the 14 days, the fish were exposed to the range of concentration reported in the polluted rivers in Niger Delta Nigeria (250, 500, and 750) mg/L and the control for 28 days. During this period, the fish were fed on 0.5% of the total body weight, and aquaria were maintained as an acclimatization period.
Five fish of equal length and weight were subjected to various concentrations of unadulterated crude oil for a total of 28 days. Freshly prepared test solutions were added regularly to maintain the concentration level after the waste was removed. The tanks were cleaned daily, and the water was changed 3 times a week and aerated using an air pump. Throughout the experiment, the test solution’s pH, temperature, total hardness, turbidity, and dissolved oxygen levels as well as the fish were monitored. Throughout all of the experimental phases, not a single mortalities occurred.
Physiological assessments
Effects of different concentrations of Crude oil (%) on behavioral pattern of Chrysichthys furcatus were assessed after 28 days exposure following the methods described by Li et al.5, with further explanation in Stephenand and Asmund6. Opercular beat frequency (OPF), tail beat frequency (TBF), swimming rate, hyperactivity, balancing, and skin color were used as indexes to assess the response of the fish to physiological stress. The degree of responses was represented accordingly.
Blood collection and analysis
After 28 days, blotting paper was used to remove slime and water from the fish’s skin. A plastic disposable syringe with a 26-gauge needle was used to draw blood from the caudal vein, which is situated at the base of the spine. Serum protein, creatine, urea, bilirubin, albumin, and globulin levels were analyzed after blood was drawn into plane bottles (without anticoagulants) and ice-covered at −20°C. Similarly, blood was kept in heparinized blood collecting vials for cortisol analysis. The collected blood was kept in ice-cold condition before analysis.
Sample analysis
Hadrochemical parameters
pH and temperature were measured using the HORIBA U10, total hardness and turbidity were determined using Geotech portable turbidity meter and SL 1000-PPA Colorimeter, respectively, while dissolved oxygen was measured using a dissolved oxygen meter.
Clinical indices
Total protein and glucose contents were determined by Krohn7 and Roe Elizabeth8 methods respectively. The serum urea and creatinine were determined using spectrophotometer methods as described by Liu et al.9 and Langenfeld et al.10, respectively. Bilirubin was estimated according to Jose et al.11. The bromocresol method Bulmer et al.12 was used to estimate albumin, and conventional equations were used to compute globulin content, that is, Globulin = Total protein – Albumin, while the serum cortisol was quantified using the ELISA technique13.
Data analysis
One-way analysis of variance (ANOVA) was used to examine the statistical significance of the differences between the treatment groups and the control using PC-STAT Statistical programs. Data were reported as mean ± SE. The significant difference was set up at P < 0.05.
Results
Physicochemical parameters
There were no statistically significant differences (P > 0.05) in the water quality measurements between the various treatments and the control. The study’s parameters were very consistent across all toxicant concentrations; the pH ranged between (6.90–7.50), temperature (26.90–27.30)°C, dissolve oxygen (8.10–8.20) mg/L, turbidity (0.21–0.27) mg/L, alkalinity (17.10–17.50) mg/L, and hardness (30.10–32.10) mg/L (Table 1).
| Parameters | pH | Temp (°C) | DO (mg/L) | Turbidity (mg/L) | Alkalinity (mg/L) | Hardness (mg/L) |
|---|---|---|---|---|---|---|
| Concentrations (mg/100 mL) | x–±SE | x–±SE | x–±SE | x–±SE | x–±SE | x–±SE |
| Control | 7.50±0.20 | 27.30±0.10 | 8.20±0.02 | 0.21±0.01 | 17.30±0.10 | 30.10±0.20 |
| 250.00 | 6.90±0.30 | 26.90±0.50 | 8.10±0.10 | 0.27±0.20 | 17.10±0.15 | 33.20±0.30 |
| 500.00 | 7.01±0.02 | 27.10±0.50 | 8.20±0.50 | 0.25±0.10 | 17.50±0.20 | 30.10±0.20 |
| 750.00 | 7.30±0.10 | 27.10±0.30 | 8.10±0.30 | 0.22±0.05 | 17.10±0.10 | 32.10±0.20 |
Physiological responses
Table 2 shows the physiological responses of C. furcatus to sublethal crude oil concentrations. As the toxicant concentration increases, so does the frequency of opercular beats. The OBF was normal in the control treatments and at the lowest dosages of 250 mg/100 mL. Similar trends were reported in the other physiological parameters except the swimming rate and balance the fish conditions were abnormal irrespective of the treatments.
| Parameters | Control | 250.00 | 500.00 | 750.00 |
|---|---|---|---|---|
| Obf | − | − | + | ++ |
| Tbf | − | − | ++ | ++ |
| Swimming rate | − | + | +++ | ++++ |
| Hyperactivity | − | − | ++++ | ++++ |
| Skin’s color | − | − | +++ | +++ |
| Balance | − | + | ++ | ++++ |
The responses of the fish were designated based on the severity (−) good condition; (+) abnormality (0 < 5%); (++), abnormality (> 5 < 10%); (+++), abnormality (> 10 < 20%), (++++), abnormality (> 20%); Obf : Opercular beat frequency; Tbf: Tail beat frequency
Clinical indices
Fish exposed to higher doses of toxicants had higher blood glucose levels, but there were no statistically significant differences (P > 0.05) between the control and experimentalgroupsexceptthatthehighestconcentrations of 750 mg/100 mL were significant (P < 0.05). The glucose induction was highest in the 750 mg/100 mL treatment and least in the 250 mg/100 mL treatment. The concentrations of the glucose in the control 250, 500, and 750 mg/100 mL crude oil treatments were; 44, 51, 53, and 69 mg/100 mL (Figure 1).
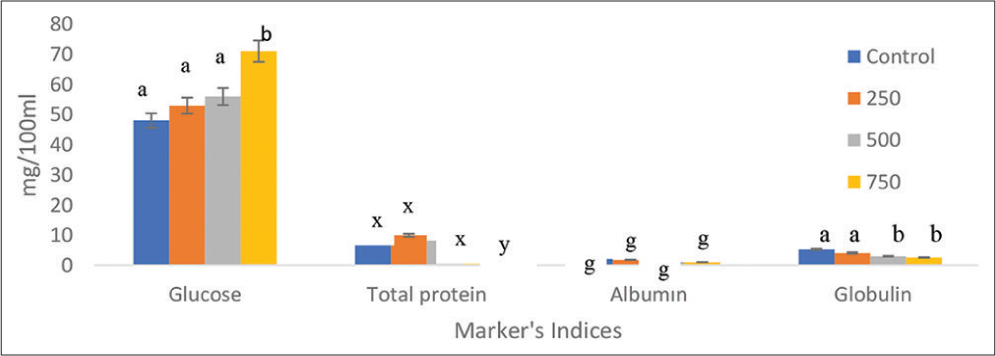
- Effects of crude oil (mg/100 mL) on blood glucose, serum protein, plasma albumin, and plasma globulin of C. furcatus at different concentrations. Data presented as mean ± SE. Significant differences between the control and experimental groups are indicated by different letters above the bars (P < 0.05).
Protein levels in the control and 250, 500, and 750 mg/100 mL of crude oil exposure were; 10, 8, 7, and 5 mg/100 mL. No significant different between the control and the various treatments in the plasma protein (P > 0.05) except the 750 mg/100 mL, where P < 0.05. Furthermore, there was significant different (P > 0.05) between other treatments and 750 mg/100 mL (Figure 1).
The albumin levels in the control and various treatments were; 0.24, 0.22, 0.18, and 0.13, respectively. There was no statistically significant difference (P > 0.05) between the control and treatment groups. Control, 250 mg/100 mL, 500 mg/100 mL, and 750 mg/100 mL of crude oil exposure resulted in globulin levels of 3.90, 3.50, 2.87, and 2.30 mg/100 mL, respectively. There was no statistically significant difference (P > 0.05) in globulin concentrations between the control group and any of the treatment groups, except for the 750 mg/100 mL group. Similarly, 750 mg/100 mL was significantly different from the other treatments (P < 0.05) (Figure 1).
Creatine levels were 3.50, 2.87, and 2.30 mg/100mL in the control and crude oil exposures of 250, 500, and 750 mg/100ml, respectively. Other than the 750 mg/100 mL treatment, there was no statistically significant (P > 0.05) difference between the control and the other treatments. Furthermore, the 750 mg/100 mL treatment was significantly (P < 0.05) different from the rest of the treatments.
The urea concentrations in the control and crude oil exposures of 250, 500, and 750 mg/100 mL were 31.0, 33.0, 33.20, and 42.30 mg/100 mL, respectively. Except for the 750 mg/100 mL, where P < 0.05, there was no significant difference between the control and the various treatments in the urea (P > 0.05). Similarly, there were significant differences (P < 0.05) between the other treatments and the crude oil treatment of 750 mg/100 mL (Figure 2).
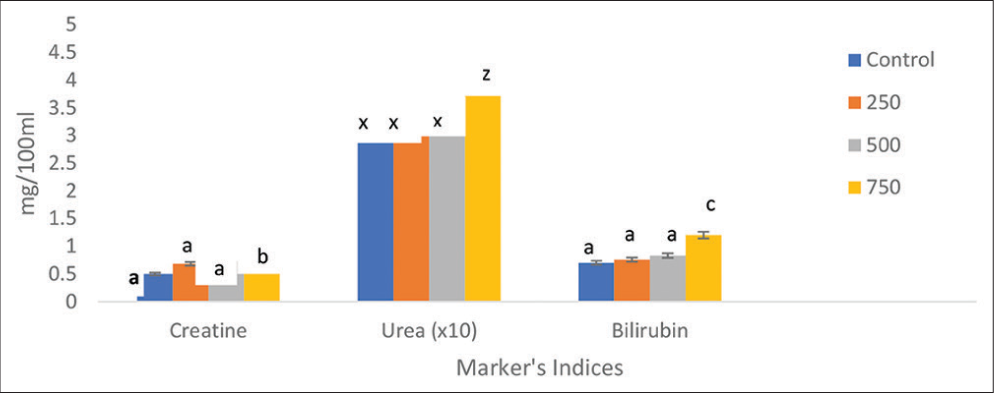
- Effects of crude oil (mg/100 mL) on creatine, urea, and bilirubin of C. furcatus at different concentrations. Data presented as mean ± SE. Different letters above the bars represent statistically significant differences between the control and experimental groups (P < 0.05).
Bilirubin concentrations in the control and crude oil exposures of 250, 500, and 750 mg/100 mL were 31.0, 33.0, 33.20, and 42.30 mg/100 mL, respectively. Except for 750 mg/100 mL, where P < 0.05, there was no significant change in bilirubin levels between the control and the various treatments (P > 0.05). Similarly, there were significant differences (P < 0.05) between the other treatments and the 750 mg/100 mL treatment (Figure 2). Plasma corticosteroid levels were 57.48, 37.17, 32.97, and 29.22 mg/100 mL in the control and crude oil exposures of 250, 500, and 750 mg/100 mL, respectively. Between the various treatments and the control, the plasma corticosteroid level fluctuates considerably and was significance at P < 0.05, but not within the treatments (Figure 3).
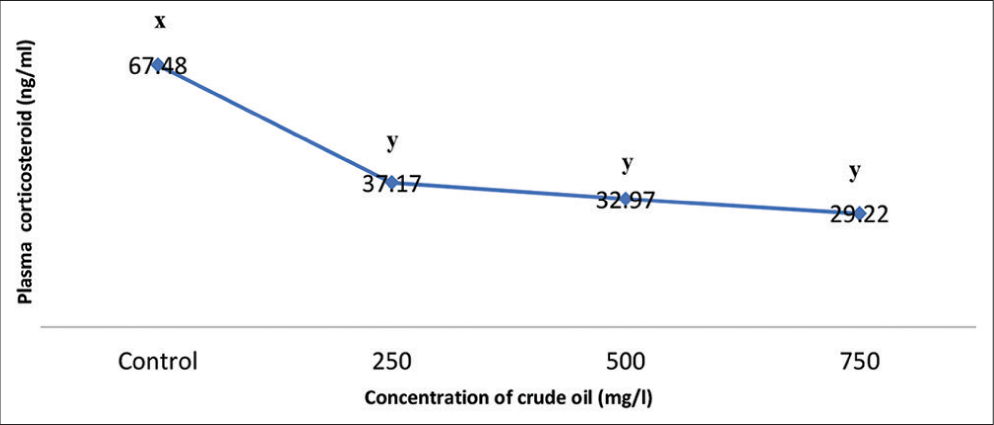
- Effects of crude oil (mg/100 mL) on plasma corticosteroid of C. furcatus at different concentrations. Data presented as mean ± SE. Significant different between the experimental and control groups are indicated by letters above the bars (P < 0.05).
Discussion
Adapting to the challenges of life in an ever-evolving environment require organisms to exhibit behaviors that allow them to respond to both external and internal inputs. It is a dynamic process, because it responds to and modifies the physical, chemical, social, and physiological aspects of its surroundings14. The fish in the control tank seemed calm and less stressed out. They were alert to any disruption with their well-synchronized motions, although their opercula activity was significantly reduced in the morning.
C. furcatus, when exposed to crude oil, exhibited disruptive behavior, irregular, erratic, and darting swimming motions, and lost balance, eventually hanging vertically in the water. An absence of coordination and avoidant behavior was also noticed.
Notable was the reaction to food, as fish in control conditions ingested feeds at predictable intervals and consistently left behind some food after each feeding. Furthermore, in the early hours, they could be seen hastily gulping the feed particles, they would quickly identify the food and approach it gently. However, fish swimming in crude oil-treated water would take longer to find the food and would then rush toward it, gulping every bit down. You never know if it will be in the morning, afternoon, or night. When considering both dosage and duration, the reactions are quite variable. According to Joachim et al.15, a similar observation was noted in rainbow trout, the fish exhibit both reactive and proactive stress management, like mammals. These results are consistent with what was found when Hülya et al.16 sentinel freshwater fish were subjected to the toxicant diazinon.
Fish blood glucose levels have been widely employed as a sensitive measure of environmental stress17, as carbohydrates are the primary and direct source of energy for fish. At the highest dosage of 750 mg/100 mL, the correlation between blood glucose and crude oil concentration was statistically significant (P < 0.05). Due to the extreme stress that pollutants place on the fish’s physiological activities, there has likely been a rise in blood sugar as a result of a higher rate of blood sugar usage to fulfill the additional energy needs imposed by the toxicant. The induction of adrenal hormones, notably glucocorticoids and Catecholamines, by crude oil may contribute to this hyperglycemic situation by causing the body to convert stored glycogen into blood glucose (glycogenolysis). Labeo rohita fishes treated with a 1ppm concentration of Phosphamidon for 15 days showed an increase in blood sugar, as reported by Christopher et al.18. The research of Sojinu et al.19 conducted on Dimethoate-treated Catla catla in the presence of pesticide toxicity, lent credence to the current results. Similarly, Akter et al.20 found that the organophosphate insecticide Phosalone induced a spontaneous elevation in blood sugar levels in the Indian main carp Labeo rohita (Hamilton). Furthermore, Sharbidre21 found that fish exposed to chlorpyrifos had higher blood glucose levels, which may be an indication that the fish need more energy to deal with the stress brought on by toxicant.
Total hypoproteinemia, hypoglobulinemia, and hypoalbuminemia were seen in the treated fishes compared to the control group; this could be because the toxicant promotes hyperactivity, leading to the breakdown of food reserves to meet energy demand Lew et al.22. For example, when carbohydrates are scarce, fish will turn to protein for fuel. Proteins are essential to cellular metabolism and regulate the process of contact between the intracellular and extracellular media22, making them the most significant and abundant macromolecules in living organisms. The decrease in total protein content caused by crude oil exposure may be attributable to the breakdown of proteins during the production of some amount of energy for the organism23. Equally, preventing protein synthesis, protein denaturation, or stoppage in amino acid synthesis could all contribute to the decline in protein levels24. According to Hossam and Fagr25, protein stores were decreased, because toxicant exposure made animals more energetic. In addition, under stressful situations, fish do not store the protein in their body tissue26, instead, the fish use their body proteins to meet their increased energy needs through a process called gluconeogenesis27.
Fish whose plasma total protein content was measured after being exposed to crude oil showed decreased albumin and globulin levels. Energy and nitrogenous metabolism during toxicant stress both come from globin and albumin, which play a major role in the structure of the cell28. Consistent with previous research on fish exposed to several toxicants; Colisa fasciatus29, the present investigation revealed that crude oil exposure significantly decreased protein levels in fish, which conforms with the observation made earlier on fish exposed to various toxicants; Colisa fasciatus29, Catla catla30, and Labeo rohita31.
The glucocorticoid cortisol controls metabolism by stimulating gluconeogenesis in the liver. By preventing nicotinamide adenine dinucleotide (NADH) from being converted to nicotinamide adenine dinucleotide (NAD+), glucose intake is also reduced. The rise in blood sugar can be traced back to both of these causes. The release of CRH and ACTH in the brain and pituitary gland serves as a negative feedback loop that controls cortisol production32. When the body is under acute or chronic stress, the anterior pituitary gland produces much more ACTH, which is then followed by a dramatic increase in cortisol secretion by the adrenal cortex. There is no stress, HPI axis dysfunction, or interrenal fatigue when cortisol levels are low33,34. Cortisol levels were found to be significantly lower in the C. furcatus study compared to the control, suggesting impairment in the HPI axis or interrenal exhaustion, which is consistent with the previous findings in Oreochromis mossambicus34. Endosulfan and heavy metal exposure were found to have similar effects on the interrenal axis by Dorval et al.35 and Satyanarayana36. In addition, Tiwari37 found that when O. mossambicus was exposed to thiodon, cortisol levels also dropped.
Conclusion
The slow metabolic rate demonstrated by an increase in glucose synthesis and a decrease in protein, albumin, globulin, and cortisol levels has been demonstrated by the fish in this investigation, showing that the fish can mount a sufficient response to a normal stressor and maintain homeostasis. Although, this attenuation may decrease the fish’s capacity to induce the metabolic and ionic adaptations required for the stress response. The fish studied has been established to be a suitable sentinel in studying anthropogenic toxicity as seen in crude oil-producing concentration-dependent behavioral abnormalities, glucose, and protein indices departure from normal settings. In treated fishes, a normal cortisol response was diminished, as was their capacity to adapt to the constant challenges provided by stressors.
Significance Statement
Petroleum is made up of aromatic hydrocarbons, which have lethal and sublethal toxic effects on aquatic life and, inevitably, public health. C. furcatus is a hardy species. In the laboratory investigation, Pisces biochemical and physiological anomalies can be used to predict what aquatic organisms will encounter in the field. The impact of the oil spill causes death and injury to many aquatic flora and fauna, which may disrupt the ecosystem and take a long time to recover to its normal state. The effects of oil spills on aquatic life and the environment are widespread and can be devastating. Ecosystem destruction is the cause of environmental degradation that may leads to emigration and extinction of some low-population species. Another issue that harmed the ecosystem was a decrease in the reproduction of many species as a result of oil’s destruction of their eggs and larvae.
References
- Polycyclic aromatic hydrocarbon (PAH) pollution and its associated human health risks in the Niger Delta Region of Nigeria: A systematic review. Environ Process. 2021;8:455.
- [CrossRef] [Google Scholar]
- Health risk assessment of the urban stretch, lower niger rivers with references to aquatic biota and anthropoids. Singapore J Emerg Res. 2021;3:104.
- [Google Scholar]
- Exposure risks topolycyclic aromatic hydrocarbons by humans and livestock (cattle) due to hydrocarbon spill from petroleum products in Niger-delta wetland. Environ Int. 2018;115:38.
- [CrossRef] [PubMed] [Google Scholar]
- Polycyclic aromatic hydrocarbons in edible mushrooms from Niger Delta, Nigeria: Carcinogenic and non-carcinogenic health risk assessment. Asian Pac J Cancer Prev. 2017;18:437.
- [Google Scholar]
- Evaluating crude oil chemical dispersion efficacy in a flow-through wave tank under a regular non-breaking wave and breaking wave conditions. Mar Pollut Bull. 2009;58:735.
- [CrossRef] [PubMed] [Google Scholar]
- The colorimetric detection and quantitation of total protein. Curr Protoc Cell Biol. 2002;Appendix 3:Appendix 3H.
- [CrossRef] [PubMed] [Google Scholar]
- Blood glucose determination: Plasma or serum. J Clin Lab Anal. 2012;26:317.
- [CrossRef] [PubMed] [Google Scholar]
- Colorimetric optoelectronic dynamics analyzer for measuring total ammonia of biological samples. IEEE J Transl Eng Health Med. 2018;5:2800610.
- [CrossRef] [PubMed] [Google Scholar]
- Colorimetric determination of urea using diacetyl monoxime with strong acids. PLoS One. 2021;16:e0259760.
- [CrossRef] [PubMed] [Google Scholar]
- The anti-mutagenic properties of bile pigments. Mutat Res. 2008;658:28.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of serum albumin concentration in healthy and diseased Hermann's tortoises (Testudo hermanni): A comparison using electrophoresis and the bromocresol green dye-binding method. J Herpetol Med Surg. 2013;23:20.
- [CrossRef] [Google Scholar]
- Behaviour surveillance and oxygen consumption in the fresh water fish Labeo rohita exposed to sodium cyanide. Biotechnol Anim Husbandary. 2010;26:91.
- [CrossRef] [Google Scholar]
- Does Individual Variation in stress response andagonistic behaviour reflect divergent stress coping strategies in Juvenile Rainbow trout. Physiol Biochem. 2005;78:715.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue-specific antioxidative and neurotoxic responses to diazinon in Oreochromis niloticus. Pestic Biochem Physiol. 2006;84:215.
- [CrossRef] [Google Scholar]
- Biochemical and morphological changes in carp (Cyprinus carpio L.) liver following exposure to copper sulfate and tannic acid. Comp Biochem Physiol C Toxicol Pharmacol. 2001;128:467.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of oil spills on fish production in the Niger Delta. PLoS One. 2018;13:10.
- [CrossRef] [PubMed] [Google Scholar]
- Polycyclic aromatic hydrocarbons in sediments and soils from oil exploration areas of the Niger Delta, Nigeria. J Hazardous Mater. 2010;174:641-7.
- [CrossRef] [PubMed] [Google Scholar]
- Toxiceffectsofanorganophosphate pesticide, envoy 50 SC on the histopathological hematological and brain acetylcholinesterase activities in stinging catfish (Heteropneustes fossilis) J Basic Appl Zool. 2020;81:47.
- [CrossRef] [Google Scholar]
- Effect of methyl parathion and chlorpyrifos on certain biomarkers in various tissues of guppy fish, Poecilia reticulate. Pestic Biochem Physiol. 2011;101:132.
- [CrossRef] [Google Scholar]
- Impact of pesticide contamination on aquatic microorganism populations in the littoral zone. Arch Environ Contam Toxicol. 2013;64:399.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of heavy metal, chromium on protein metabolism in brain and muscle of freshwater fish, Channa striatus. Int J Curr Microbiol App Sci. 2016;5:638.
- [CrossRef] [Google Scholar]
- Concentration distribution and ecological risk assessment of polycyclic aromatic hydrocarbon in Kolo Creek Niger-Delta Nigeria. Sci Rev. 2020;6:62.
- [CrossRef] [Google Scholar]
- Study the effect of hexavalent chromium on some biochemical, citotoxicological, and histopathological aspects of Orechromis spp. Fish. Pak J Biol Sci. 2007;10:3973.
- [CrossRef] [PubMed] [Google Scholar]
- Genotoxic effects of chlorpyrifos in freshwater fish Cirrhinus mrigala using micronucleus assay. Adv Biol. 2016;17:105.
- [CrossRef] [Google Scholar]
- Effect of chlorpyrifos and monocrotophos on locomotor behavior and acetylcholinesterase activity of subterranean termites, Odontotermes obesus. Pest Manage Sci. 2004;60:986.
- [Google Scholar]
- Changes in growth performance and biochemical status of fresh water fish Cirrhinus mrigala exposed to sublethal doses of chlorpyrifos. Int J Agric Food Sci Technol. 2014;2:619.
- [Google Scholar]
- Biochemical markers of contamination in fish toxicity tests. Interdiscip Toxicol. 2011;4:85.
- [CrossRef] [PubMed] [Google Scholar]
- Distributions and potential sources of polycyclic aromatic hydrocarbons in surface sediments from an emerging industrial city (Xinxiang) Environ Monit Assess. 2016;188:61.
- [CrossRef] [PubMed] [Google Scholar]
- The toxicity effect of monocrotophos 36% E.C on the biochemical changes Labeo rohita (Hamilton 1882) Int J Sci Res Dev. 2016;3:11.
- [Google Scholar]
- Effects of synthetic pyrethroids cyfluthrin and fenvalerate on nucleic acid contents of fresh water fish Gambusia affinis. Int J Sci Res Biol Sci. 2019;6:20.
- [CrossRef] [Google Scholar]
- Biochemical responses of Clarias gariepinus to cypermethrin. Environ Anal Toxicol. 2014;5:1.
- [Google Scholar]
- The morphological, physiological, histopathological and biochemical response of some non-target organisms to the stress induced by the pesticides in the environment, Pesticides In: Anthropogenic activities and Health of our Environment. Vol 28. London: IntechOpen; 2019.
- [Google Scholar]
- Impact of cypermethrin on fingerlings of edible carp Labeo rohita. Sci World J. 2012;7:6.
- [CrossRef] [PubMed] [Google Scholar]
- Biochemical alterations as total proteins (Tp), aspartate aminotransferases (AAT) and alanine amino transferases (ALAT) induced by permethrin (A synthetic pyrethroid of Type I) in the fish Ctenopharyngodon idella (GRASS CARP) Int J Recent Sci Res. 2019;10:34397.
- [Google Scholar]
- Impact of cypermethrin on fingerlings of common edible carps (Labeo rohita) Sci World. 2012;29:1395.
- [CrossRef] [PubMed] [Google Scholar]






