Translate this page into:
Relation of Serum Adenosine Deaminase to Some Risk Factors in Type 2 Diabetes Mellitus
*Email: drashokkumarj@gmail.com
Abstract
Aim:
Study was conducted to compare and correlate the levels of serum adenosine deaminase and serum uric acid with Fasting Plasmaglucose (FPG), Post Prandial Blood Sugar (PPBS) and glycated hemoglobin in type 2 diabetes mellitus.
Materials and Method:
Study group consisted of 60 diabetics and 50 age and sex matched healthy individuals. Blood sample was collected in fasting state and two hours after the food for estimation of serum Adenosine Deaminase (ADA), uric acid, parameters of lipid profile, fasting and postprandial glucose.
Result:
An increased level of serum ADA, FPG, PPBS, parameters of lipid profile and glycated haemoglobin in type 2 diabetes mellitus compared to controls. A statistically significant correlation was seen between serum ADA as well as serum uric acid with, Fasting Plasma Glucose (FPG), Post Prandial Blood Glucose (PPBS), glycated haemoglobin. Serum ADA had shown significant correlation with the Triglyceride (TG), Total Cholesterol (TC), Low Density Cholesterol (LDL cholesterol). A negative correlation was seen between serum ADA acid and High Density Lipoprotein cholesterol (HDL cholesterol).
Conclusion:
Adenosine deaminase and serum uric acid levels increased and positively correlated with parameters of lipid profile and glycemic control. This indicates that ADA and serum uric acid can serve as predictor of glycaemic index and help to monitor the long term effects of diabetes mellitus.
Keywords
Adenosine Deaminase
Diabetes Mellitus Type 2
Glycated Haemoglobin
Introduction
Diabetes mellitus is most common endocrine disorder with multiple aetiologies. The pathophysiology of hyperglycaemia is changed from triumvirate to ominous octet to dirty dozen. Long term hyperglycaemia leads to glucotoxicity which further leads to poor glycaemic control and predisposes to long term micro and macro vascular complications. Hence more number of parameters are required which can elicit the risk of complications in diabetes.
Adenosine deaminase (EC 3.5.4.4) is involved in catabolism of adenosine and deoxyadenosine. It deaminates adenosine and deoxyadenosine, which is later converted to hypoxanthine. Hypoxanthine can be further converted to uric acid by xanthine oxidase or can be salvaged1. Uric acid is present as sodium urate in the extracellular fluid. Epidemiological studies had established the relationship between the serum uric acid and the increased risk of cardiovascular disease2. Under normal conditions uric acid acts as anti-oxidant. But under selected metabolic conditions it acts as prooxidant3. The relationship between uric acid and the cardiovascular disease is debatable.
Even though factors like serum TG, TC, FPG and PPBS are increased in hypertriglyceridemia, the relationship of hyperuricemia with these factors is not establish4.
ADA has been shown to impair the insulin sensitivity for glucose transport and anti-lipolytic action by inactivating extracellular adenosine, which adipocytes release spontaneously5. ADA activates lipolysis and markedly potentiates the increase in cyclic AMP accumulation due to norepinephrine6. Dysregulated fat metabolism and consequent elevation of free fatty acids leads to subsequent development of type 2 diabetes mellitus. Serum adenosine deaminase levels are increased in type 2 diabetes mellitus.
The aim of the present study was, to evaluate the serum adenosine deaminase and serum uric acid level levels in type-2 diabetics by comparing and correlating with FPG, PPBS, glycated haemoglobin, HDL, LDL and TG to establish its role in glycemic control and role in risk for coronary artery disease.
Materials and Methods
Study group was selected from individuals visiting endocrinology outpatient department (with proven diabetes mellitus type 2 on treatment) and for general health check-up. The total number of subjects included in the study were 110. Of which 60 were diabetics and 50 were age and sex matched controls. Controls were selected from individuals who came to hospital for heath check-up. Individuals with no abnormal findings on physical examination and normal laboratory parameters were included in the control group.
Subjects with findings suggestive of tuberculosis, leprosy, acute lymphadenitis, Hodgkin’s, hepatitis A and B, lymphoma enteric fever, chicken pox were excluded from the study group. Approval was granted by the Institutional ethical committee for the study.
After ensuring that patient was fasting for 8 to 10 hours, the informed consent was obtained for collection of blood samples. By a septic precaution 2 ml each of venous blood was collected in a sugar tube, plain tube and an EDTA tube. Sugar tube was processed to obtain plasma for estimation of glucose. Blood collected in plain was immediately processed and serum was obtained. Serum was used for the estimation of serum ADA, TG, TC and HDL cholesterol. EDTA sample is used for estimation of glycated haemoglobin. Exactly 2 hours after a stomach full of breakfast 2 ml of blood was collected for estimation of PPBS.
Giusti and Galanti colorimetric method was used for estimations serum ADA7. Glycated haemoglobin was measured by ion exchange resin by using commercially available kit8. Serum TC was estimated by enzymatic method CHOD-PAP (Cholesterol Oxidase-Peroxidase 4-Aminoantipyrine) in semi autoanalyzer using commercially available kit9. Serum TG was measured by enzymatic method GPO-ESPAS (Glycerol 3 Phosphate Oxidase Peroxidase N-Ethyl-N-Sulfopropyl-n-anisidine) using commercially available kit in semi-autoanalyser10. Serum HDL cholesterol was measured by enzymatic method after precipitation with polyanions in semi-autoanalyzer using commercially available kit11. Serum LDL cholesterol was calculated by using Friedewald formula (LDL cholesterol mg/dl = Total cholesterol – (HDL Cholesterol + Triglycerides/5))11. Caraway method was used for estimation of serum uric acid12.
Results
The percentage distribution of the parameters measured in the present study is shown in Figure 2. We observed 90% of the diabetics had glycated haemoglobin level above 12 percent and 10% of the diabetics had glycated haemoglobin between 10 to 12 percent. Fasting plasma glucose was above 160 mg/dl in 98.3% of the diabetics of the study group and 1.7% had fasting glucose between 100 mg/dl to 160 mg/dl. In all diabetics of the study group had PPBS above 200 mg/dl.
We observed a statistically significant (p<0.001) increase of serum ADA in diabetes mellitus type 2 in comparison with the control group (Table 1). Serum levels of TG, TC, LDL cholesterol and HDL cholesterol levels were significantly increased in cases when compared to controls (Figure 1). Serum ADA level had shown a significant (p < 0.001) positive correlation with glycated haemoglobin, FPG, PPBS, LDL cholesterol, TG and negative correlation with HDL cholesterol (Table 2).
| Study group | Serum ADA (U/L) |
|---|---|
| Type 2 diabetes mellitus | 52.71 ± 5.95 |
| Controls | 17.00 ± 4.18 |
| P value | <0.001 |
P value <0.005 – significant
| Parameters Correlated | Cases | Control | ||
|---|---|---|---|---|
| r-value | p-value | r-value | p-value | |
| Serum ADA and glycated haemoglobin | 0.985 | <0.001 | 0.625 | <0.001 |
| Serum ADA and FPG | 0.778 | <0.001 | 0.707 | <0.001 |
| Serum ADA and PPBS | 0.669 | <0.001 | 0.416 | <0.001 |
| Serum ADA and LDL | 0.953 | <0.001 | 0.388 | <0.001 |
| Serum ADA and TG | 0.858 | <0.001 | 0.765 | <0.001 |
| Serum ADA and HDL | -0.604 | <0.001 | -0.416 | <0.001 |
P value <0.005 – significant

- Serum levels of TG, TC, LDL cholesterol and HDL cholesterol in cases and controls.
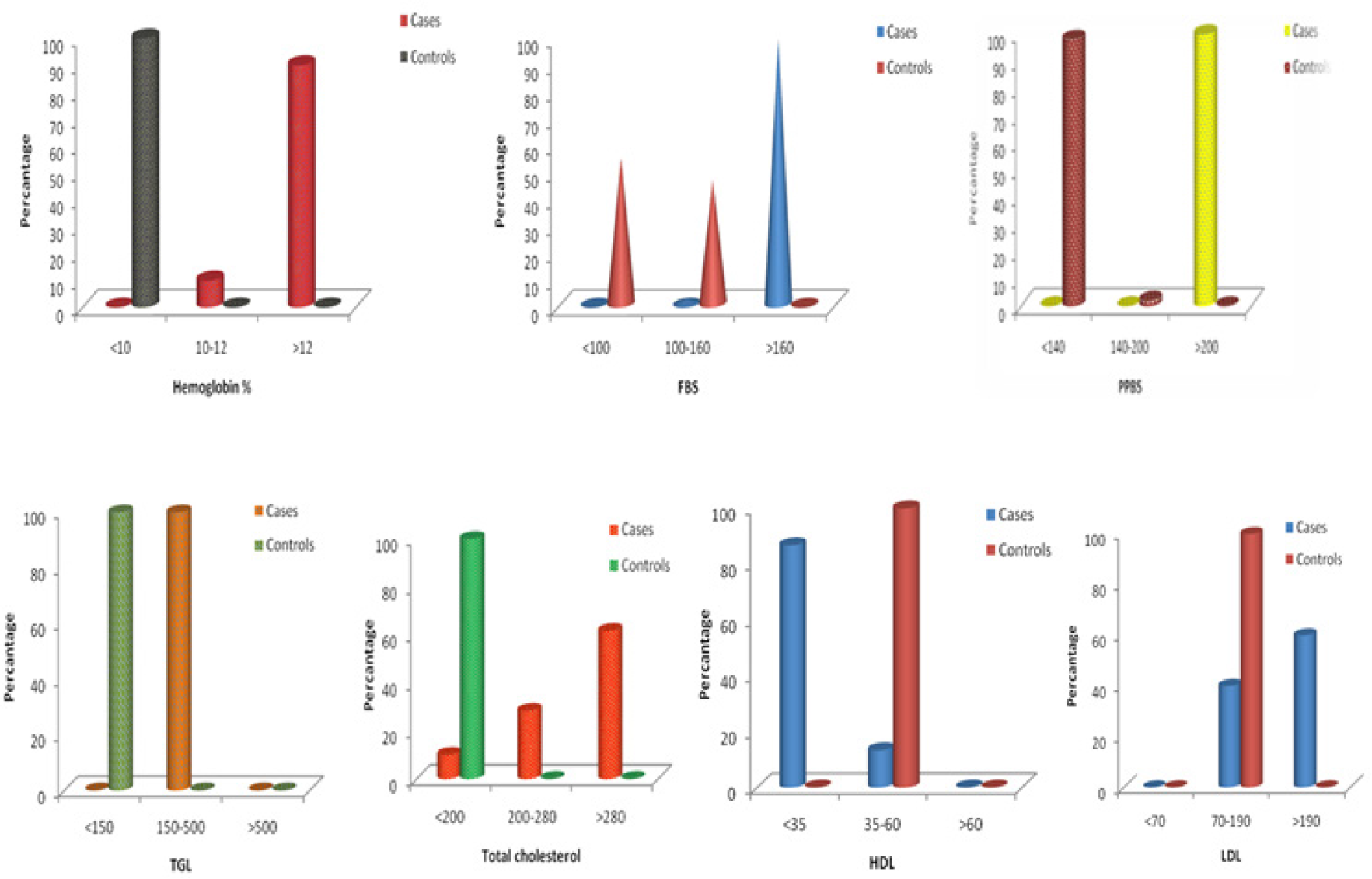
- Percentage distribution of HbA1c, FBS, PPBS, TGL, TC, HDL, LDL in cases and controls.
Serum uric acid level was significantly (p < 0.001) increased in cases (5.86 ± 0.46 mg/dl) when compared to controls (4.35 ± 0.45 mg/dl) (Table 3). Pearson correlation had shown statistically significant (p < 0.001) positive correlation between serum uric acid and glycated haemoglobin, FBS PPBS, whereas the same observation was not made in control group (Table 4).
| Study Group | Serum Uric Acid (mg/dl) |
|---|---|
| Type 2 diabetes mellitus | 5.86±0.46 |
| Controls | 4.35±0.45 |
| P value | <0.001 |
| Parameters Correlated | Cases | Control | ||
|---|---|---|---|---|
| r-value | p-value | r-value | p-value | |
| FBS vs. Uric acid | 0.680 | <0.001 | -0.100 | 0.488 |
| PPBS vs. Uric acid | 0.596 | <0.001 | -0.043 | 0.765 |
| Glycated haemoglobin % vs. Uric acid | 0.872 | <0.001 | -0.031 | 0.831 |
Discussion
The relationship between the serum adenosine deaminase and the serum uric acid with the glycemic parameters (glycated haemoglobin, FBS, PPBS) and parameters of lipid profile (TG, TC, LDL cholesterol, HDL cholesterol) in diabetes mellitus type 2 was evaluated. There was a significant (p < 0.01) increase in serum ADA in individuals with diabetes mellitus type 2 in comparison with control group. This is consistent with the study of Siddiqui et al. They also have observed elevated serum ADA in diabetes mellitus type 213. S. M. Dasegowda, et al had shown that a positive correlation is present between serum ADA and oxidative stress14. This indicates that as the serum ADA level increases there is increase in oxidative stress, which can contribute to chronic complications of diabetes mellitus type 2. We observed that there is a positive correlation between the glycated haemoglobin and ADA, which indicate that as the glycated haemoglobin increases serum ADA level also increases. This finding was in accordance with the study of Shinde et al15. Kurtul et al also demonstrated a significant correlation between serum ADA and HbA1c16. Glycated haemoglobin is used as predictor of glycemic control, poor glycemic control showed increase in ADA levels. Havilah et al demonstrated increase in ADA in diabetes mellitus type 2 and can be considered as an independent marker of glycemic status17.
We observed statistically significant positive correlation between ADA and serum TG, TC and LDL cholesterol. There was a significant negative correlation between ADA and HDL cholesterol. In type 2 diabetes mellitus dyslipidaemia was observed. Tagoe et al had made similar observation of dyslipidaemia in diabetes mellitus type 218. Dyslipidaemia on a long term causes cardiovascular complications. This study therefore indicates that ADA can be used as an indicator to predict long term cardiovascular complications in diabetes mellitus type 2.
Serum uric acid level is elevated in diabetes mellitus type 2 compared to control group. There was a significant correlation between serum uric acid and indicators of glycemia. Uric acid although generally considered to be an important antioxidant, experimental and human studies demonstrated that it has pro-oxidant activity19.
A. Kaur et al had shown that there is an increase in serum ADA as the glycated hemoglobin level increased and increase in serum uric acid when there is moderate increase in HbA1c (<7%), but further increase (>7%) in HbA1c they found decrease in serum uric acid levels20. But we found that 90% of the cases had glycated hemoglobin more than 12%, showing a positive correlation with serum uric acid levels.
Uric acid level above the upper 1/3rd of the normal range (>4 mg/dl) considered as one of the multiple factors that contribute to endothelial dysfunction. In hyperuricemia Endothelial Nitric Oxide Synthase (eNOS) is uncoupled. Endothelium becomes a net producer of reactive oxygen species, especially superoxide anion when. The dysfunctional endothelium associated with type 2 DM produces reactive oxygen species12. Vascular smooth muscle proliferation is promoted by uric acid21.
Conclusion
Adenosine deaminase and serum uric acid levels were increase in type 2 diabetes mellitus. There was a positive correlation between adenosine deaminase, serum uric acid and the indictors of glycemic control in type 2 diabetes mellitus. Parameters of lipid profile are increased in diabetes mellitus type 2. Serum TG, total cholesterol and LDL cholesterol are positively correlated with serum adenosine deaminase. The study showed positive correlation between glycated haemoglobin, FBS and PPBS. It is important to detect complications early and a single marker is required to identify. Hence ADA and uric acid level may be used as indicators of glycaemia and risk for atherosclerosis.
Acknowledgement
We than the individuals willingly participated in the study and the A.J. Institute of Medical Sciences and Research Centre for all the support.
References
- Adenosine deaminase In: Review of physiological roles. Czech Republic: University of South Bohemia; 2001.
- [Google Scholar]
- Hyperuricemia and increased risk of ischemic heart disease in a large Chinese cohort. International Journal of Cardiology. 2012;154(3):316-21.
- [CrossRef] [PubMed] [Google Scholar]
- Uric acid puzzle: Dual role as anti-oxidant and pro-oxidant. Electrolytes and Blood Pressure. 2014;12(1):1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological association between uric acid concentration in plasma, lipoprotein (a), and the traditional lipid profile. Clinical Cardiology. 2010;33(2):E76-80.
- [CrossRef] [PubMed] [Google Scholar]
- Enhancement by Adenosine of insulin-induced activation of phoshoinositide 3-kinase and protein kinase B in rat adipocytes. Journal of Biological Chemistry. 1999;274:1945-50.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis and glucose metabolism of fat cells. The Journal of Biological Chemistry. 1975;250(3):1027-34.
- [CrossRef] [Google Scholar]
- Adenosine deaminase. In Methods of Enzymatic Analysis New York: Academic Press; 1974. p. :1092-9.
- [CrossRef] [Google Scholar]
- The clinical information value of glycated haemoglobin assay. New Eng J Med. 1984;310:341-6.
- [CrossRef] [PubMed] [Google Scholar]
- Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470-5.
- [CrossRef] [Google Scholar]
- Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476.
- [CrossRef] [Google Scholar]
- Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clinical Chemistry. 1983;29(12):2026-30.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of uric acid in serum by a carbonate method. American Journal of Clinical Pathology. 1955;25(7):840-5.
- [CrossRef] [PubMed] [Google Scholar]
- A study on the modulation of Adenosine Deaminase (ADA) activity in monocytes of type 2 diabetic patients by antioxidants. Indian Academy Journal of Clinical Medicine. 2011;12(2):113-6.
- [Google Scholar]
- Serum adenosine deaminase as oxidative stress marker in type 2 diabetes mellitus. International Journal of Research in Medical Sciences. 2015;3(5):1195-8.
- [CrossRef] [Google Scholar]
- Evaluation of oxidative stress in type 2 diabetes mellitus and follow-up along with vitamin E supplementation. Ind J Clin Biochem. 2011;26(1):74-77.
- [CrossRef] [PubMed] [Google Scholar]
- Adenosine deaminase activity in the serum of type 2 diabetic patients. Acta medica (Hradec Kralove)/Universitas Carolina. Facultas Medica Hradec Kralove. 2003;47(1):33-5.
- [CrossRef] [Google Scholar]
- Adenosine deaminase activity in type-2 diabetes mellitus-an independent marker of glycemic status and stimulator of lipid peroxidation. International Journal of Chemical and Life Sciences. 2013;2(06):1175-8.
- [Google Scholar]
- Type 2 diabetes mellitus influences lipid profile of diabetic patients. Annals of Biological Research. 2013;4(6):88-92.
- [Google Scholar]
- Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Experimental Nephrology. 2012;121(3-4):e71-8.
- [CrossRef] [PubMed] [Google Scholar]
- Serum adenosine deaminase activity and its correlation with glycated haemoglobin levels in patients of type 2 diabetes mellitus. Journal of Clinical and Diagnostic Research. 2012;6(2):252-6.
- [Google Scholar]
- Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutrition and Metabolism. 2004;1(1):1.
- [Google Scholar]






