Translate this page into:
Receptor Mediated Effect of Melatonin in Pituitary Regulated Thyroid Secretions of Hyperthyroid Mice
*Email: shivssbhu@yahoo.co.in
Abstract
The present study is designed to delineate the receptor mediated effect of melatonin in pituitary gland regulated thyroid secretions of hyperthyroid mice. Immuno histochemical study has been done to observe the melatonin receptors localization in pituitary gland. Western blot analysis was carried out to assess the expression patterns of melatonin receptors in pituitary gland and TSH receptors in thyroid gland. Hormonal analysis was done by ELISA kits. It was observed that introduction of melatonin reduced serum T3, T4, TSH hormones levels including thyroid TSH receptors those were fluctuated due to experimentally induced hyperthyroidism. The result has also shown the depletion of MT1 and MT2 receptors of pituitary gland due to melatonin treatment in hyperthyroidism. It may conclude that melatonin effects in pituitary regulated thyroid secretions by down regulation of pituitary MT1 and MT2 receptors in hyperthyroid condition.
Keywords
Melatonin
Melatonin Receptors
Pituitary
Thyroid Hormones
TSH Hormone
TSH Receptor
Introduction
The production and secretion of thyroid hormones are triggered by the anterior pituitary gland secretion Thyroid Stimulating Hormone (TSH). TSH hormones bind on TSH-receptor on thyroid epithelial cells that seems to stimulate all of the processes necessary for thyroid hormones production. The hyperthyroidism refers to any condition in which there is too much thyroid hormone produced in the body. Hyperthyroid condition is characterized by an increased metabolism including the disruption of other normal activities of the physiological system. This abrupt situation negatively influenced different activities of the body including the functions of cardiac tissue by elevation of the lipid peroxidation1, 2.
Melatonin (N-acetyl-5-methoxytryptamine) is the main secretory product of the pineal gland also produced in the various extra-pineal organs where it may signal in autocrine or paracrine modes2, 10, 14, 15. Melatonin is a well known antioxidant and free radical scavenger, widely distributed in the organism. Mutual relationships between the pineal secretion melatonin and the thyroid have for a long time been a subject of intensive research. Melatonin plays an important protective role against various thyroid abnormal situations. Melatonin may protect against oxidative damage in the course of certain thyroid disorders. Oxidative damage and the protective action of melatonin may occur during both physiological and pathological processes in the thyroid5.
Melatonin performs various functions through binding with target tissues’ MT1 and MT2 receptors. According to the available study, vasoconstrictor and vasodilation performance of melatonin may lead through activation of MT1 and MT2 receptors respectively8. In few cases such as scavenging activities of melatonin for reactive oxygen and nitrogen species were done by non-receptor mediated way3.
Evidences were available regarding the receptors mediated as well as receptors non-mediated action of melatonin during different physiological and pathological conditions excluding hyperthyroidism. Thus, the current study was designed to evaluate the effect of melatonin in pituitary regulated thyroid hormones, T3 and T4 secretions followed by the TSH receptor expressions of thyroid gland of hyperthyroid mice. This effect of melatonin was observed with concurrent observations of localization and expressions of melatonin receptors in pituitary gland.
Materials and Methods
Mice experimental investigations were taken according to the revised Animal Act (2007) of Animal Welfare, Government of India.
Animal Procurement and Maintenance
Five mice (n = 5) were colonized at each polycarbonate cage (43 cm x 27 cm x 14 cm) to avoid the crowding effect. The experimental animals were settled at animal house with suitable laboratory temperature of 25+2°C and maintained 12 hrs light and 12 hrs dark cycles. Mice feed and sufficient water was provided to the mice for their proper health growth.
Experimental Design
For analyzing the receptor mediated actions of melatonin in pituitary regulated thyroid hormones secretions in hyperthyroidism, experimental animals were categorized into four groups, five mice (n = 5) were settled for each experimental group.
Control (Con) group: Saline treatment (0.01%), 0.1 ml/day was given to this group of mice.
Melatonin (Mel) group: melatonin was provided subcutaneously to this group of mice at the concentration of 25 µg/100 g body weight per day for consecutive 30 days at evening time, 16:30 hrs-17:00hrs.
Hyperthyroid (T4) group: L-thyroxine was provided subcutaneously to this group of mice at the concentration of 0.6 µg/g body weight per day for consecutive 30 days.
(T4 + Mel) group: Both L-thyroxine and melatonin were given to this group of mice consecutively for 30 days.
Sample Collection and Processing
Experimental animals were anaesthetized and after sacrificing them blood serum was collected from blood samples of each group. Collected blood serum were kept at -20oC until analyzed. The part of pituitary and thyroid glands were dissected out from each groups of mice and stored at freeze (-20oC) for performing western blotting for melatonin receptors of pituitary and TSH receptors of thyroid gland. The part of each tissue from each experimental group was fixed in Bouin’s fixative for performing immunohistochemistry.
Hormonal Analysis
Commercial ELISA kits (Diagnostic AutomatationInc, CA, USA) were used for analyzing the hormones (T3, T4 and TSH) concentrations.
Immunohistochemical Staining
Immunohistochemical staining was done to observing the localization of melatonin receptors11 in pituitary gland. 5mm thin paraffin sections were kept on slides coated by 3% gelatin. Alcohol grades were used over them for deparaffinising and rehydrating. Then tissue sections were taken in PBS and then kept in methanol (with 0.3% H2O2) for blocking the activity of peroxide. Sections washed with PBS (0.1M Na2HPO4, NaH2PO4, 0.9% NaCl, pH=7.4) and then for 2 hrs kept in blocking solution. The sections were incubated with primary antibodies of melatonin receptors at 4oC overnight. Next day sections were washed by PBS for 3 times and incubated with secondary antibody. After this incubation the sections were again washed for 3 times by PBS and then incubated to preformed AvidinBiotin reagent for 30 minutes. The immune interactions were detected by using DAB in in 0.01 M Tris-Cl (pH = 7.6) and 0.1% H2O2 and counterstained with Ehrlich’s haematoxylin. The samples were dehydrated, mounted by DPX and observed followed by taking microphotographs under 40 X of Olympus microscopes BX 41.
Western Blot analysis
Experimental tissue samples were homogenized lysed and then quantified the proteins in the studied samples by following Lowry method. SDS-PAGE was used for resolving aliquots containing 100 mg proteins followed by electro transfer to nitrocellulose membrane (Santa Cruz Biotech, USA). Primary antibodies of MT1, MT2 and TSH-R were taken for immune detection. Antibody of β-actin was taken and dissolved in PBS containing 5% skimmed milk and 0.01% Tween-20. That was followed by incubation with secondary antibodies conjugated with horse-radish peroxidase. Super Signal West Pico Chemiluminescent Substrate was used for detecting the immune responsiveness. The protein bands were quantified by measuring the optical density by Scion Image Analysis Software. Expression of value (% control value) was measured by taking the ratios of the specific signal with the signal of β-actin16.
Statistical Analysis
Data was analyzed by ANOVA and experimental results were taken significant by Tukey’s HSD multiple range test. For calculating the data and for preparing the graph Microsoft Excel program and SPSS were obtained.
Results
Serum T3 level
Significant (p<0.01) decrease of T3 hormone level was observed in melatonin injected mice group, whereas, it was found significantly (p<0.01) increased in L-thyroxine induced hyperthyroid group of mice as compare to control group of mice. Melatonin administration in hyperthyroid condition in mice significantly (p<0.01) decreased the T3 hormone concentration as compare to hyperthyroid group of mice (Figure 1).

- T3 hormone level of blood serum of different experimental groups of mice. Mean ± SEM was represented by Histogram. The experimental changes were significantly considered when p< 0.05. **p<0.01: Control group vs. Melatonin group, Control group vs. T4-treated (hyperthyroid) group; ##p<0.01: T4-treated (hyperthyroid) group vs. T4-treated (hyperthyroid) +Melatonin treated group.
Serum T4 level
In melatonin treated mice, T4 hormone level was significantly (p<0.01) decreased and in L-thyroxine induced hyperthyroid group of mice, significant (p<0.01) increase of T4 level was noted in comparison to control mice group. But in hyperthyroid along with melatonin treatment group, significant (p<0.01) decrease of T4 concentration was observed as compare to hyperthyroid mice group (Figure 2).
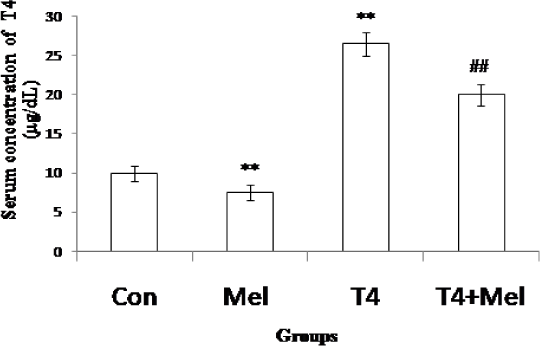
- T4 hormone level of blood serum of different experimental groups of mice. Mean ± SEM was represented by Histogram. The experimental changes were significantly considered when p<0.05. **p<0.01: Control group vs. Melatonin group, Control group vs. T4-treated (hyperthyroid) group; ##p<0.01: T4-treated (hyperthyroid) group vs. T4-treated (hyperthyroid) +Melatonin treated group.
Serum TSH level
The level of TSH was remain unchanged in melatonin treated mice whereas it was found significantly (p<0.01) increased in hyperthyroid group of mice as comparison with control mice. But in hyperthyroid along with melatonin treatment group, TSH level was found to be decreased significantly (p<0.01) as compare to hyperthyroid mice group (Figure 3).
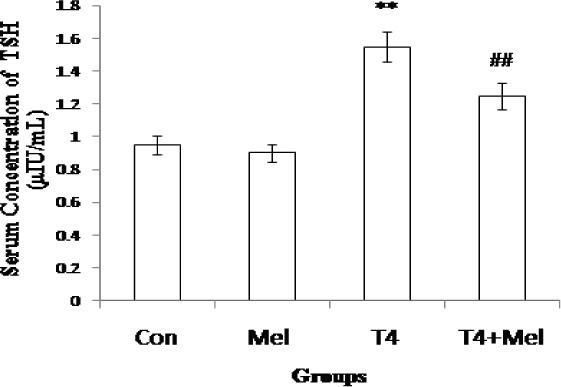
- TSH hormone level of blood serum of different experimental groups of mice. Mean ± SEM was represented by Histogram. The experimental changes were significantly considered when p<0.05. **p<0.01: Control group vs. Melatonin group, Control group vs. T4-treated (hyperthyroid) group; ##p<0.01: T4-treated (hyperthyroid) group vs. T4-treated (hyperthyroid) + Melatonin treated group.
TSH Receptor Protein Expression in Thyroid Gland
Significantly (p<0.01) increased expression of TSH receptor was observed in melatonin treated mice group and also in hyperthyroid mice group as compare to control mice group. But in hyperthyroid along with melatonin injected group of mice, TSH receptor expression was observed significantly (p<0.01) decreased in comparison to hyperthyroid mice group (Figure 4).
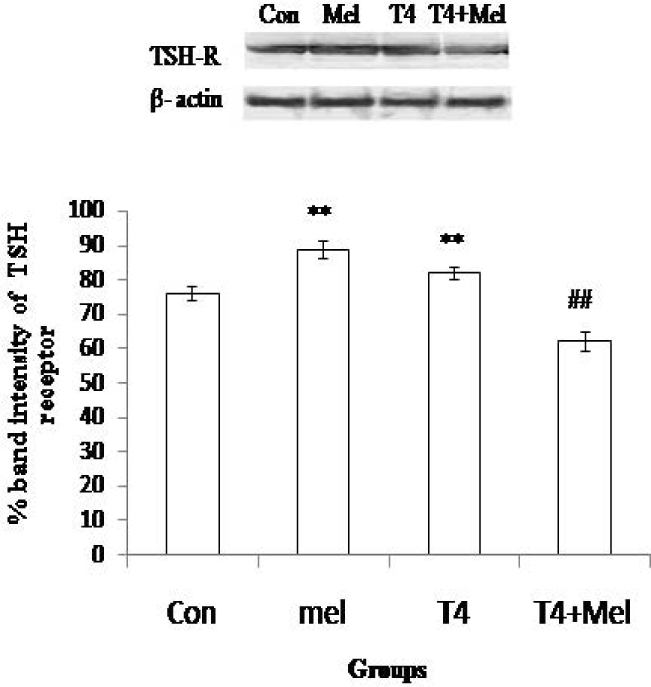
- TSH receptor protein expression of pituitary gland. As loading control β-actin was used. Percent expressions of proteins were shown at lower panel done by Scion Image analysis software. Mean ± SEM was represented by Histogram. The experimental changes were significantly considered when p<0.05. **p<0.01: Control group vs. Melatonin group, Control group vs. T4-treated (hyperthyroid) group; ##p<0.01: T4-treated (hyperthyroid) group vs. T4-treated (hyperthyroid) +Melatonin treated group.
Immunohistochemical Observation of MT1 and MT2 Receptors in Pituitary Gland
In pituitary gland, MT1 and MT2 receptors immunoreactivity were observed in pars distalis region as shown (Figure 5).

- Immunohistochemistry of MT1 receptor (a) and MT2 receptor (b) of pituitary gland, observed under microscope in 40X magnification. In control (c) section, DAB was not detected. Microphotographs were taken by Olympus Microscope.
MT1 Receptor Protein Expression in Pituitary Gland
In both melatonin treatment group and hyperthyroid group of mice, MT1 receptor protein expression of pituitary was noted significantly (p<0.01) increased as compare to control group of mice. But in hyperthyroid along with melatonin administered group of mice, MT1 expression of pituitary was found to be decreased significantly (p<0.01) in comparison to L-thyroxine treated hyperthyroid mice group (Figure 6).
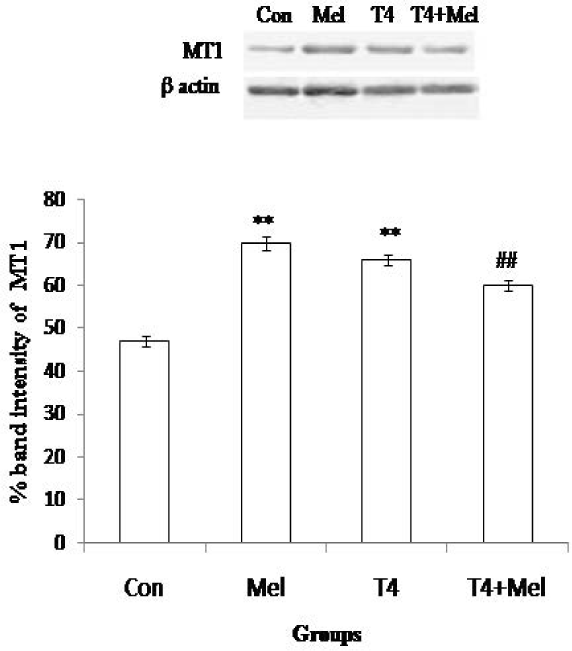
- MT1 receptor protein expression of pituitary gland. As loading control β-actin was used. Percent expressions of proteins were shown at lower panel done by Scion Image analysis software. Mean ± SEM was represented by Histogram. The experimental changes were significantly considered when p<0.05. **p<0.01: Control group vs. Melatonin group, Control group vs. T4-treated (hyperthyroid) group; ##p<0.01: T4-treated (hyperthyroid) group vs. T4-treated (hyperthyroid) +Melatonin treated group.
MT2 Receptor Protein Expression in Pituitary Gland
MT2 receptor protein expression of pituitary was significantly (p<0.01) increased in melatonin treated mice and also in L-thyroxine administered hyperthyroid mice group as compare to control. Whereas, MT2 expression of pituitary was noted significantly (p<0.01) reduced in hyperthyroid along with melatonin treated mice group in comparison to hyperthyroid mice group (Figure 7).
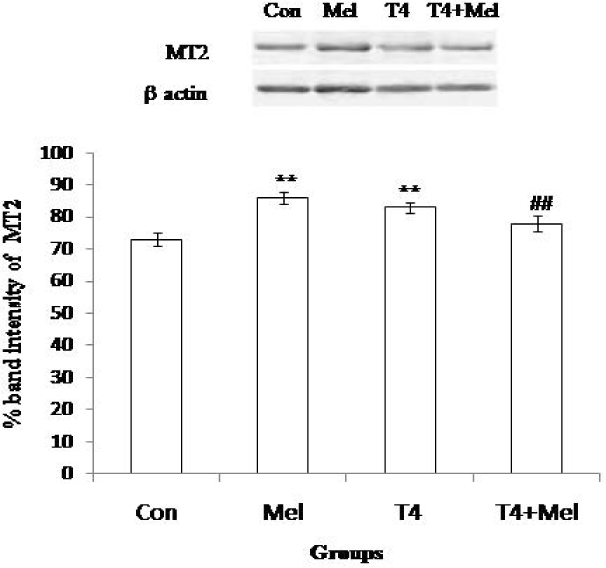
- MT2 receptor protein expression of pituitary gland. As loading control β-actin was used. Percent expressions of proteins were shown at lower panel done by Scion Image analysis software. Mean ± SEM was represented by Histogram. The experimental changes were significantly considered when p<0.05. **p<0.01: Control group vs. Melatonin group, Control group vs. T4-treated (hyperthyroid) group; ##p<0.01: T4-treated (hyperthyroid) group vs. T4-treated (hyperthyroid) +Melatonin treated group.
Discussion
The physiological disruption of thyroid gland causes the functional impairment of various organs of the body. Hyperthyroidism is one such pathological situation characterized by overt activities of thyroid gland. The interrelationship between pineal product melatonin and thyroid function have been clarified through many investigations. The present study was designed to acquire more knowledge in this field by investigating the action of melatonin in pituitary regulated thyroid productions of hyperthyroid condition in mice. In this study, introduction of melatonin causes depletion of thyroid gland secretions, T3 and T4. The inhibitory action of melatonin on thyroid gland was well established by many researchers. Supplementation of melatonin also reduced the thyroid hormone level of male rats as observed1. A high dose of L-thyroxine leads to the development of hyperthyroidism. In this study we introduced the same dose of L-thyroxine causing the high levels of thyroid hormones, T3 and T4 indicating the experimentally induced hyperthyroidism. But the injection of melatonin decreased the thyroid hormones concentrations of hyperthyroid mice. The same depletion of thyroid hormones due to melatonin during hyperthyroidism was reported by another investigator7. Melatonin supplementation in hyperthyroid condition suppresses secretion of thyroid hormones as reported9.
Administration of melatonin increased the abundance of thyroid TSH receptor proteins and possessed no impact on the pituitary secretion, TSH level. In mammalian system including human, treatment of melatonin could not change the level of TSH as reported13. In experimentally induced hyperthyroidism, significant increase of TSH hormone and thyroid TSH receptor expression were observed as compare to control. But administration of melatonin decreased the circulatory TSH hormone and thyroid TSH receptor expression. Melatonin may influence the thyroid secretions (T3 and T4) through inhibited the production of TSH secreted from pituitary gland as well as by down regulation of thyroid TSH receptors.
Melatonin modulates different physiological actions through mediation of its MT1 and MT2 receptors. Immunohistochemical study revealed melatonin receptors, MT1 and MT2 localization in the pars distalis region of pituitary gland. Many other studies also suggested the presence of (I125) iodo-melatonin binding sited in anterior pituitary. Cell specific expression of MT1 mRNA in pars distalis of embryonic pituitary was also documented in rat4. Both MT1 and MT2 melatonin receptors expression in pituitary gland increased in melatonin administered group and also in L-thyroxine induced hyperthyroid group as compare to control group. Introduction of melatonin and thyroxine alone induced the up regulation of MT1 and MT2 receptors of pituitary. In our previous laboratory studies alterations of melatonin receptors were observed in other endocrine tissues in hyperthyroid condition of mice6. Several reports cited by other investigators have also pointed on the alterations of melatonin receptors in the hypothalamic SCN during depression17. But treatment of hyperthyroid along with melatonin reduced the expression of MT1 receptor as well as MT2 receptor expression. Melatonin may impact on pituitary gland in hyperthyroid condition through down regulation of its receptors.
Conclusion
Above findings suggest the inhibitory action of melatonin on thyroid secretions by controlling over the pituitary gland in hyperthyroid condition through suppression of pituitary secreted thyroid stimulating hormone and thyroid stimulating hormone receptor proteins expression of thyroid. This kind of modulation occurred by melatonin over pituitary gland through down regulation of MT1 and MT2 receptors. Overall it may conclude the important intra-pituitary physiological role of melatonin in the regulation of thyroid gland secretions. Although further studies are need to attempt to elucidate the molecular cell level pathway of melatonin with the help of its receptors association in this facet.
Acknowledgment
We would like to thank University Grants Commission (UGC) and Council of Scientific and Industrial Research (CSIR) for their financial assistance. We would like to express our gratitude to State Biotech Hub, Tripura University, sponsored by DBT for facilitating the laboratory equipments.
References
- Opposite effects of zinc and melatonin on thyroid hormones in rats. Toxicology. 2004;195(1):69-75.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal melatonin: Localization, function and clinical relevance. Digestive Diseases and Sciences. 2002;47(10):2336-48.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin in diseases of the oral cavity. Oral Diseases. 2010;16(3):242-7.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of MT1 melatonin receptor expression in the foetal pituitary. Journal of Neuroendocrinology. 2006;18(1):50-6.
- [CrossRef] [PubMed] [Google Scholar]
- The role of oxidative stress in physiological and pathological processes in the thyroid gland; possible involvement in pineal-thyroid interactions. Neuro Endocrinology Letters. 2003;24(5):293-303.
- [Google Scholar]
- Melatonin modulates thyroid hormones and splenocytes proliferation through mediation of its MT1 and MT2 receptors in hyperthyroid mice. Proceedings of the Zoological Society. 2018;71(2):186-93.
- [CrossRef] [Google Scholar]
- The interaction of oxidative stress response with cytokines in the thyrotoxic rat: Is there a link? Mediators of Inflammation. 2009;2009:391682.
- [CrossRef] [PubMed] [Google Scholar]
- MT2 melatonin receptors are present and functional in rat caudal artery. Journal of Pharmacology and Experimental Therapeutics. 2002;302(3):1295-302.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of intraperitoneal melatonin supplementation on the release of thyroid hormones and testosterone in rats with hyperthyroid. Neuro Endocrinology Letters. 2003;24(5):345-7.
- [Google Scholar]
- Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Progress in Neurobiology. 2008;85(3):335-53.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of melatonin MT1 receptor immunore-activity in human retina. Journal of Histochemistry and Cytochemistry. 2002;50(4):519-26.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid peroxidation levels in rat cardiac muscle are affected by age and thyroid status. Journal of Endocrinology. 2000;164(1):97-102.
- [CrossRef] [PubMed] [Google Scholar]
- Lack of changes in serum prolactin, FSH, TSH and estradiol after melatonin treatment in doses that improve sleep and reduce benzodiazepine consumption in sleep-disturbed, middle-aged and elderly patients. Journal of Pineal Research. 2001;30(1):34-42.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinology Metabolism. 2008;19(1):17-24.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin: A hormone, a tissue factor, an autocoid, a paracoid and an antioxidant vitamin. Journal of Pineal Research. 2003;34(1):75-8.
- [CrossRef] [PubMed] [Google Scholar]
- Antiestrogens modulate MT1 melatonin receptor expression in breast and ovarian cancer cell lines. Oncology Reports. 2006;15(1):231-5.
- [CrossRef] [PubMed] [Google Scholar]
- Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. Journal of Affected Disorders. 2013;148(2-3):357-67.
- [CrossRef] [PubMed] [Google Scholar]






