Translate this page into:
Prevention of Mother-to-Child Transmission of HIV in Delta State of Nigeria: Evaluation of the Early Infant Diagnosis Program
*Email: enwose@csu.edu.au
Abstract
Background:
The global public health issue of Mother-to-Child Transmission (MTCT) of HIV is being addressed with Prevention of MTCT (PMTCT) strategy. A narrative review has recently been published.
Objective:
The aim of this study was to determine from an early infant diagnosis program in Delta State if there is reduction in MTCT of HIV.
Methods:
This was a retrospective study. Three health institutions comprising of one from each of the three senatorial district in Delta State were used for the study. The records of 1080 prenatally exposed babies that had DNA PCR tests between 6 weeks to 18 months were evaluated descriptively. Data mining was from the period of August 2009 to December, 2016. Child follow-up register was reviewed for information that included, age the child had PCR done, commencement of Antiretroviral Therapy (ART) at birth, if mother was on ART, result of PCR and test done at 18 months.
Results:
98.0% of HIV positive mothers were on ART during pregnancy, 98.9% of the infants were on ART at birth, and 93.1% were exclusively breast fed. The outcome of the PMTCT program obtained shows that compared to 100% effectiveness among infants who were compulsorily treated on the basis of their mothers being on treatment, there is 89% efficiency among those who either themselves or their mothers received treatment; but only 17% efficiency was achieved where neither mother nor child is receiving treatment.
Conclusion:
This study shows that with effective PMTCT intervention, reducing MTCT of HIV is being achieved by the public health service.
Keywords
Early Identification
Infant HIV
PMTCT Efficiency
Introduction
The agenda for Mother-to-Child Transmission (MTCT) of Human Immunodeficiency Virus (HIV) revolves around the vertical infection of HIV from mother to her child1. MTCT is by far the most common way that children become infected with HIV2. MTCT of HIV is known to be able to occur any time from conception to delivery and breastfeeding3. The World Health Organization (WHO) guidelines recommend that pregnant mothers who are HIV-positive should be treated with a regimen of three Antiretroviral drugs (ARV) as soon as possible- and stay on these drugs until their infant is born and breast-feeding concluded4,5.
The prevalence of MTCT is up to 45%; but it is believed that this rate of infection can be reduced to below 5% with effective interventions6–8, which include prevention of vertical infection during pregnancy and appropriate breastfeeding practices9. MTCT of HIV is one of the biggest challenges of the HIV/AIDS management especially in resource constrained settings10–12. Africa has the highest burden of the disease accounting for about 90 percent of paediatric HIV infections13–15. In 2009 approximately 370,000 children became infected with HIV globally and most of these infections occurred through MTCT15.
It is acknowledged that Nigeria accounts for 30% of the burden of MTCT of HIV in the world14,16. This justifies why Nigeria is one of the 22 focus countries of the Global Plan to Eliminate MTCT1,17,18. Though the Nigerian Government has implemented PMTCT of HIV program, evidence on the effectiveness of PMTCT remains limited14. Yet, evidence of the effectiveness of PMTCT has been clearly demonstrated in large scale programs that are integrated into routine antenatal care19.
Early Infant Diagnosis (EID) is part of the PMTCT program. This involves EID testing directly for HIV DNA by Polymerase Chain Reaction (PCR) method. EID makes it possible for early identification and referral of HIV-positive infants. This enables the infants to receive early clinical evaluation and antibiotic prophylaxis against opportunistic infections as well as Antiretroviral Therapy (ART)20–23. Furthermore, a review of HIV test results in an EID program provides a unique opportunity for evaluating the success of the PMTCT program and reducing MTCT14.
Thus, this research seeks to examine an EID program in Delta State of Nigeria with a view to determine the level of reduction of MTCT of HIV. A narrative literature review has previously been performed as background to this work16. Another study has also examined EID program in two other Southern States of Nigeria14. Through descriptive statistics, the study will evaluate the effectiveness of the PMTCT interventions program in Delta state, Nigeria and any challenges in follow-up. Specifically, the aim of this study is to determine the:
Effectiveness of PMTCT of HIV program.
Challenges of follow-up of care for HIV-positive mothers and their exposed babies.
Methods
Study Design and Setting
This was a descriptive retrospective study aimed at evaluating data from early infant diagnosis program in Delta State, Nigeria. Delta State consists of 25 local government areas. The state had three national senatorial districts-Delta South, Delta North and Delta Central. The study was conducted in the three senatorial district- Delta South, Delta North and Delta Central. For the purpose of the study, the purposive sampling method was adopted in the selection of three facilities comprising one per senatorial district. Hence the selected facilities were the Central Hospitals at (1) Agbor in Delta North Senatorial District, (2) Ughelli in Delta Central Senatorial District, and (3)Warri in Delta South Senatorial District. Similar to the method of Anoje et. al, (2012), data inclusion criteria were based on the HIV exposed infants being aged six weeks to 18 months, Dried Blood Spot (DBS) samples taken for a DNA PCR tests and a complete set of record available.
Data Collection
The information was collected through data mining of the archived records. Specifically, the primary data source of collection was hospital records, which were stored in hard copy register. The data were retrieved from medical record department with the support of medical record officer and PMTCT Focal Person. All data were de-identified by the data miners such that no set of information is traceable to any individual. Data collected included information on infants and their mothers as follows
Infants: age, gender, dates of tests, results of tests, HIV drug, age-at-testing, type of feeding, outcome of infant at eighteen months.
Mothers: if mother was on HIV drugs or not.
Ethical Considerations
The facilities were clearly informed about the study in order to gain their consent. Anonymity, privacy, and confidentiality were maintained throughout the study. This was achieved by a letter of introduction for the commencement of the study which was written to the ethical board of the respective hospitals and ministry of health Delta State. Ethics approvals were given by Delta State hospital management board (Protocol number CHW/ECC VOL 1/145) and the Ministry of Health (Ref number: HM/596/154) before the study was conducted. Letter of introduction was collected from Department of Public and Community Health, College of Health Sciences, Novena University Ogume, Delta State.
Data Analysis
Microsoft excel spreadsheet and SPSS version 21 were used for data management and statistical analysis, respectively. This was designed to be a descriptive study without hypothesis, and as such, analysis was done using frequency counts and percentage.
Limitations of the Study
The selection of only three sites that offer EID services in the State may have led to bias. Two of the facilities used could not provide records from the beginning of the program. Some available records were not properly documented.
This study was done as a Master of Public Health dissertation, which was bound by time limit. Getting ethical approval from the respective hospitals was a big problem. It took more than 6 weeks to get both ethical approvals from Ministry of Health Asaba and Central Hospital Warri. Approval from Ministry of Health Asaba was obtained on 27th of October 2017 and that of Central Hospital Warri was obtained on 2nd of November 2017. Although, the medical record officer and PMTCT focal person made it possible for quick collection of data for analysis to meet up with the school’s set-time, this was only possible about 2 to 3 weeks to the deadline of submission of the project work. As result of the delay, there was no time to crosscheck or make further investigations on the data collected, especially to know the reasons for loss to follow up.
Results
From August 2009 to December 2016 of the DBS records, a total sample size of 1080cases that had a well-documented data was collected for analysis. Beside the ‘N=1080’; another thirteen infants and their mothers were not on ART at time of first test, which were included in assessment of PMTCT efficiency.
The results show that 49.8% of the babies were males, 98.0% of the mothers were on anti-retroviral drugs while 2.0% (22 mothers) of them were not. For the infants, 98.9% of them were on anti-retroviral drugs while 1.1% (12 infants) of them was not – thus a total of 25 infants were not on treatment at time of first test. Further, 93.1% of the infants were on exclusive breastfeeding while 6.3% were not breast feed at all (Table 1). In terms of age at first test, 87.9% of the infants had their first DBS samples collected within the age of 6 weeks to 5 months while 0.7% were collected at 15 months or more (Figure 1).

- Frequency bar chart showing the age of infants at time of 1st BDS tested.
| Measures | Response | Frequency | Percentage |
|---|---|---|---|
| Number of infants tested* | 1080 | ||
| Mothers on anti-retroviral drug | Yes | 1058 | 98 |
| Infants on anti-retroviral drugs | Yes | 1068 | 98.9 |
| Type of infant feeding | Exclusive breastfeeding | 1005 | 93.1 |
| Mixed feeding | 7 | 0.6 | |
| Nil breastfeeding | 68 | 6.3 |
On analysis of Question 1 – evidence of reduction in MTCT of HIV: result shows that 95.6% of the infant tested negative at first screening test. At 18 months follow-up, 95.6% of the infants were apparently healthy, 3.8% were referred forART, 0.4% died, while 0.3% were lostto follow up (Table 2).
| Frequency | Percentage | ||
|---|---|---|---|
| Infantstested at first (N = 1080) | Positive | 48 | 4.4 |
| Negative | 1032 | 95.6 | |
| Apparently healthy* | 1032 | 95.6 | |
| Findings at follow-up(N = 1080) | Referred to ART** | 41 | 3.8 |
| Dead | 4 | 0.4 | |
| Lost cases | 3 | 0.3 |
Among the 25 infants who were not on treatment at time of first test; they were categorized into dichotomous groups based on mothers treated vs. untreated. In a reverse analysis, 22 mothers were not on treatment at time of first infant test; and were also categorized into dichotomous groups based on infants treated versus untreated. The result of analysis shows 100% effectiveness among infants who were compulsorily treated and less than 20% where neither mother nor child is receiving treatment.
The level of negative results is the same at 18 months relative to results of tests done before 1 year (Figure 2).
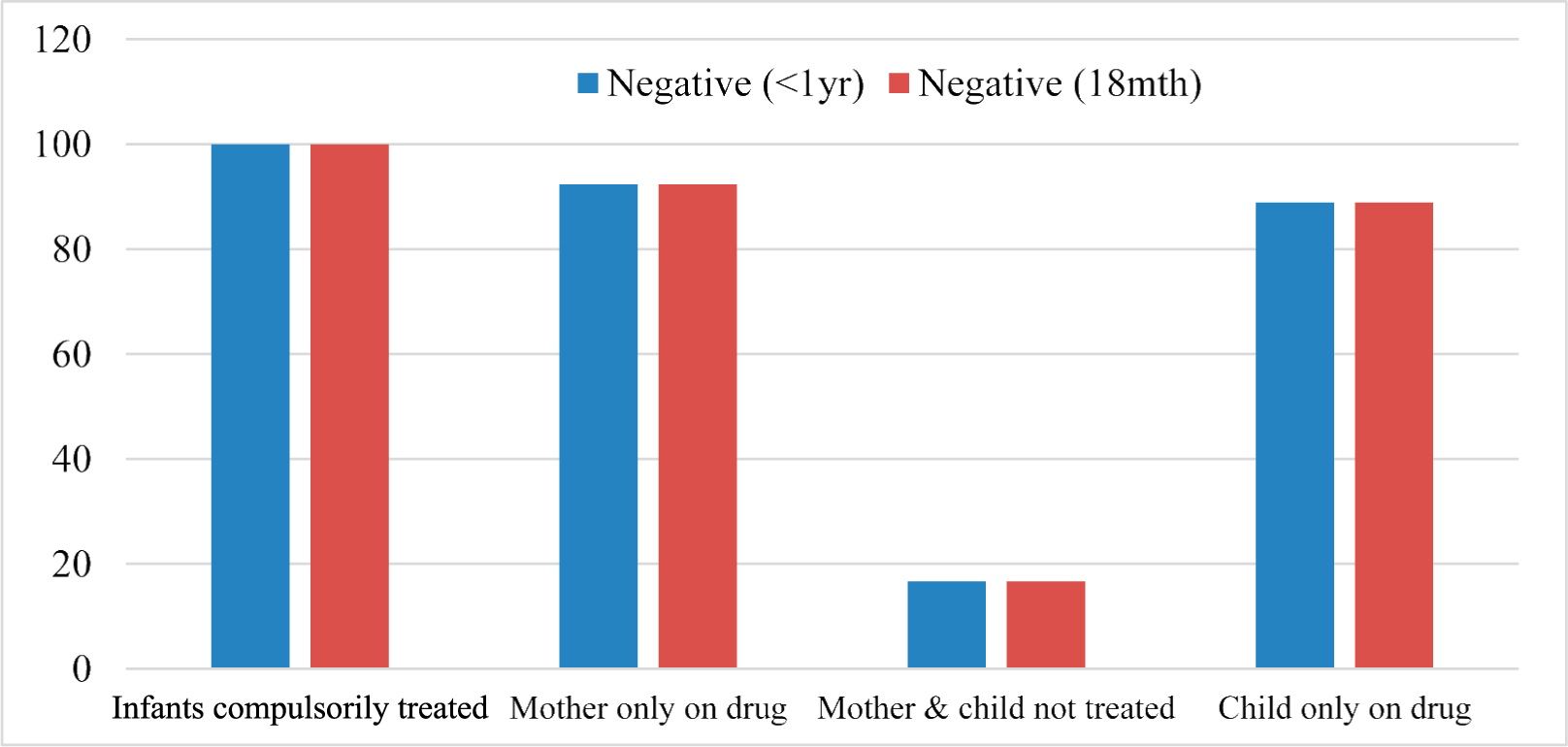
- Evaluation of treatment efficiency based on negative results in subpopulation
Discussion
Effective PMTCT program requires mother-infant pair to have access to a cascade of interventions beginning with HIV counseling and testing at the antenatal clinic; receiving ART prophylaxis if they are HIV positive; and follow-up on safe infant feeding practices14. In this study, result obtained shows that 2% of the mothers did not receive ART prophylaxis for PMTCT (Table 1). These mothers did not seek antenatal care in the facilities that have PMTCT program as such they were not counseled and tested during antenatal period. In Delta State, Government owned hospital integrated PMTCT into the regular antenatal clinic. All expectant mothers seeking antenatal care receive Provider Initiated Testing and Counseling (PITC). This is in line with the national guidelines on PMTCT of HIV in Nigeria24. This finding compares reasonably well relative to the report of attendance and acceptance of PMTCT in Zambia that support mass therapy as a policy25, and Ivory Coast where more than 80% mother-and-baby pair were on ARV prophylaxis26.
In this study, 98.9% of the exposed babies were placed on anti-retroviral drugs within 72 hours of birth till 6weeks before the first PCR test is done at 6 week while 1.1% of the exposed babes were not on drugs. This is a reflection of the level of utilization of PMTCT into regular antenatal clinic (Table 1). However, this is better than the report from another part of the Southern Nigeria where over 33% of the mothers-infant pair did not receive any ART for PMTCT of HIV14.
Results from this study reveal that 87.9% of the infant had their first DBS sample collected within the age of 6 weeks to 5 months (Figure 1). In Nigeria, the national PMTCT guidelines stipulate that the first DNA PCR test should be conducted at six weeks of age for all exposed infants14. However, it was observed that over 50% of the infants came for DBS test after 6 weeks. This is similar to findings where over 70% of infant had their first PCR done after 6 weeks14.
The result obtained from this study reveals that 95.6% of infant tested negative to HIV using DNA PCR within 6 weeks to 5 months. This finding is in agreement with the report of a related study done at Asaba in Delta State of Nigeria which showed 86.3% of babies’ HIV status based on PCR test were negative27. This result also indicates that there is reduction in MTCT of HIV in Delta State. Further, the general outcome of the exposed infant shows that 95.5% of the infants were well and alive at 18 months and they tested Negative to HIV. Among the remaining 4.5%, recorded indicated that 0.4% died before the 18 months probably due to positive HIV complications, 0.3% were lost to follow up after the first PCR test done; while the rest 3.8% (41 cases) positives were all duly referred to ART clinic as at 18th month for further management in line with the PMTCT guideline in Nigeria (Table 2).
There has been expectation that perinatal HIV remission and cure is possible in infants28, and few cases are beginning to emerge29. In this study, 7% (3/41; Table 2) of the positives that were re-tested at the 18th month appeared equivocally negative and earmarked for further retesting. A related study on children with HIV in South Africa showed that early diagnosis and ART-intervention can enhance reduction in early infant mortality rates and viral load to 7.7% and 81.4% respectively. Hence it is cautioned that interruptions to ART should be undertaken with care30–32.
The negative results obtain at the 6 weeks and 18th month appears to be the same for all the infants who were not on treatment at time of first test (Figure 2). The result of analysis shows two striking observations. First is that compared to 100% effectiveness among infants who were compulsorily treated on the basis of their mothers being on treatment, there is 89% efficiency among those who either themselves or their mothers received treatment; but only 17% where neither mother nor child is receiving treatment. Second is the level of negative results (i.e., efficiency).While 4.4% of infants on ART tested positive at first test with indication of reduction at 18th month (Table 2), up to 83% of the subpopulation of ‘mother-and-child’ who were suspected to miss early start of PMTCT (i.e., indicated by not being on ART) tested positive at both initial and 18th month tests. Other studies have previously acknowledged or implied the effectiveness of PMTCT services in Nigeria33–36, and availability of the service in Delta State16. What this report adds to the body of literature is evidence of efficiency of the EID program in Delta State.
It is observed during this study that the data were not properly documented and some of the report tools cannot be found. All documentation was on paper and none was on electronic soft copy format. It is known that data collection, analysis, and usage constitute part of the ongoing challenges confronting the processes of monitoring and evaluation of PMTCT program37. In this regard, follow-up of participants is problematic38, and the challenge of poor record keeping constitutes a major barrier39. While it has been acknowledged that improvement in recording of PMTCT data is imperative40, this report highlights the need in Delta State of Nigeria.
Conclusion
The results from this study demonstrate that there is reduction in MTCT of HIV in Delta State and that EID program is an effective PMTCT intervention. There is indication that testing and counseling of the expectant mother in antenatal clinic, as well as early initiation of ART to HIV positive mother and compulsory treatment of the exposed infant are practices that enhance success. This implies that effective tracking and monitoring of mother-baby pair is very important to PMTCT, which in turn calls for the need to improve on documentation protocols and resources. Public health officers can use this report as educational tool to intensify awareness campaign on the level of efficiency of the PMTCT. The public health officers may need to be equipped to scale up the campaign to the general public. Further, PMTCT services should be expanded to Primary Health Centers to enable pregnant mothers to access the service, especially in rural communities.
References
- Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants.
- [Google Scholar]
- New data on the prevention of mother-to-child transmission of HIV and their policy implications.
- [Google Scholar]
- Consolidated Guidelines on the use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach Geneva: World Health Organization; 2013.
- [Google Scholar]
- Higher risks of mother-to-child HIV transmission in countries with lower HIV prevalence: UNAIDS 2013 results for 32 countries with generalised epidemics. Journal of Virus Eradication. 2015;1(4):257-63.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of mother to child transmission of HIV infection in Pacific countries. Internal Med J. 2007;37(4):216-23.
- [CrossRef] [PubMed] [Google Scholar]
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach (2nd ed). 2016.
- [Google Scholar]
- Coming to terms with complexity: A call to action for HIV prevention. The Lancet. 2008;372(9641):845-59.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with HIV positive sero-status among exposed infants attending care at health facilities: a cross sectional study in rural Uganda. BMC Public Health. 2018;18(1):139.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges and opportunities of optimal breastfeeding in the context of HIV option B+ guidelines. BMC Public Health. 2017;17(1):541.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20(10):1447-50.
- [CrossRef] [PubMed] [Google Scholar]
- Reducing mother-to-child transmission of HIV:findings from an early infant diagnosis program in south-south region of Nigeria. BMC Public Health. 2012;12:184.
- [CrossRef] [PubMed] [Google Scholar]
- 4. The burden of HIV/AIDS: Implications for African States and societies. Preparing for the Future of HIV/AIDS in Africa: A Shared Responsibility Washington (DC): National Academy of Sciences (US); 2011.
- [Google Scholar]
- Reducing the mother-to-child transmission of HIV: findings from an early infant diagnosis program in Delta state Nigeria. Int J Community Med Public Health. 2017;4(12):4352-7.
- [CrossRef] [Google Scholar]
- Countdown to zero - global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive.
- [Google Scholar]
- Eliminating mother-to-child transmission of the human immunodeficiency virus in sub-Saharan Africa: The journey so far and what remains to be done. Journal of Infection and Public Health. 2016;9(4):396-407.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of a city-wide program to prevent mother-to-child HIV transmission in Lusaka, Zambia. AIDS. 2005;19(12):1309-15.
- [CrossRef] [PubMed] [Google Scholar]
- Early infant diagnosis of HIV-1 infection in Luanda, Angola, using a new DNA PCR assay and dried blood spots. . 2017;12(7):e0181352.
- [CrossRef] [PubMed] [Google Scholar]
- Cost-effectiveness of early infant HIV diagnosis of HIV-exposed infants and immediate antiretroviral therapy in HIV-infected children under 24 months in Thailand. PLoS One. 2014;9(3):e91004.
- [CrossRef] [PubMed] [Google Scholar]
- Follow-up of infants diagnosed with HIV-Early Infant Diagnosis Program, Francistown, Botswana, 2005-2012. MMWR Morbidity and Mortality Weekly Report. 2014;63(7):158-60.
- [PubMed] [Google Scholar]
- Mother-to-child transmission of HIV: Findings from an early infant diagnosis program in Bertoua, Eastern Cameroon. The Pan African Medical Journal. 2013;15:65.
- [CrossRef] [PubMed] [Google Scholar]
- National Guidelines on Prevention of Mother to Child Transmission (PMTCT) of HIV in Nigeria (4th ed). 2010.
- [Google Scholar]
- Zambian women's attitudes toward mass nevirapine therapy to prevent perinatal transmission of HIV. Lancet. 2001;358(9293):1611-2.
- [CrossRef] [PubMed] [Google Scholar]
- Operational issues in preventing mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire, 1998-99. Bull World Health Organ. 2001;79(7):641-7.
- [PubMed] [Google Scholar]
- Utilization of HIV and AIDS mother-to-child transmission prevention and babies' outcome in Asaba, Nigeria. Journal of Nursing Education and Practice. 2014;4(2):178-85.
- [CrossRef] [Google Scholar]
- Advances and hope for perinatal HIV remission and cure in children and adolescents. Current opinion in pediatrics. 2016;28(1):86-92.
- [CrossRef] [PubMed] [Google Scholar]
- Child living with HIV maintains remission without drugs since 2008: U.S. Department of Health and Human Services. 2017
- [Google Scholar]
- Outcomes of the South African national antiretroviral treatment programme for children: The IeDEA Southern Africa collaboration. South African Medical Journal-Suid-Afrikaanse tydskrif vir geneeskunde. 2009;99(10):730-7.
- [PubMed Central] [Google Scholar]
- Impact of earlier combination antiretroviral therapy on outcomes in children. Curr Opin HIV AIDS. 2015;10(1):12-7.
- [CrossRef] [PubMed] [Google Scholar]
- Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis. 2015;61(7):1169-78.
- [CrossRef] [PubMed] [Google Scholar]
- Optimizing PMTCT service delivery in rural North-Central Nigeria: Protocol and design for a cluster randomized study. Contemp Clin Trials. 2013;36(1):187-97.
- [CrossRef] [PubMed] [Google Scholar]
- Dual indices for prioritizing investment in decentralized HIV services at Nigerian primary health care facilities. Health Policy and Planning. 2016;31(3):377-89.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of a HIV prevention of mother to child transmission program in a nigerian early infant diagnosis centre. Niger Med J. 2014;55(3):204-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative effectiveness of congregation-versus clinic-based approach to prevention of mother-to-child HIV transmission: Study protocol for a cluster randomized controlled trial. Implement Sci. 2013;8:62.
- [CrossRef] [PubMed] [Google Scholar]
- Confronting challenges in monitoring and evaluation: Innovation in the context of the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Journal of Acquired Immune Deficiency Syndromes (1999). 2017;75(Suppl 1):S66-S75.
- [CrossRef] [PubMed] [Google Scholar]
- Service uptake and performance of the prevention of mother-to-child transmission (PMTCT) programme in Ibadan, Nigeria. Afr J Med Med Sci. 2010;39(2):81-7.
- [PubMed] [Google Scholar]
- Prevention of Mother to Child Transmission of HIV (PMTCT) programme in KwaZulu-Natal, South Africa: an evaluation of PMTCT implementation and integration into routine maternal, child and women's health services. Trop Med Int Health. 2010;15(9):992-9.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges with routine data sources for PMTCT programme monitoring in East Africa: Insights from Tanzania. Global health action. 2015;8:29987.
- [CrossRef] [PubMed] [Google Scholar]






