Translate this page into:
Impact of vitamin D on secretion and action on insulin: A glimmer of hope for the global burden of diabetes mellitus
* Corresponding author: Dr. Mainul Haque, The Unit of Pharmacology, Faculty of Medicine and Defence Health, Universiti Pertahanan Nasional Malaysia, Kem Perdana Sungai Besi, Kuala Lumpur, Malaysia. runurono@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ahmad R, Haque M. Impact of vitamin D on secretion and action on insulin: A glimmer of hope for the global burden of diabetes mellitus. J Health Sci Res. 2024;9:1–6. doi: 10.25259/JHSR_50_2023
Vitamin D is referred to generally as ergocalciferol (vitamin D2), found in plants, while vitamin D3, also known as cholecalciferol, is found in animal sources. Vitamin D, in small amounts, is obtained from diet; however, the major portion of vitamin D in circulation is acquired from the skin when cholecalciferol is formed from 7-dehydrocholesterol upon exposure to sunlight rays.[1] Cholecalciferol is then biotransformed to 25-hydroxycholecalciferol in the hepatic cells and then, in the kidney, turns into 1,25-dihydroxycholecalciferol.[2,3] 25-hydroxycholecalciferol [25(OH)D] remains in the circulation and is a biomarker for the level of vitamin D.[4]
Vitamin D has its role in absorbing Ca2+, growth, and remodeling of bone.[5] This vitamin also influences the immune system and biotransformation process. It has been noted in several research works that vitamin D affects the activity islets of Langerhans and resistance of insulin in type 2 diabetes mellitus (T2DM).[6–9] The increasing global trends in diabetes mellitus (DM) incidence are perhaps related to the pervasiveness of vitamin D insufficiency.[10–12]
VITAMIN D AND ISLETS OF LANGERHANS FUNCTIONING
T2DM is expected to rise globally in the decades to come. In T2DM, there is a characteristic decrease in the synthesis and release of insulin from pancreatic islets and the development of resistance to insulin. Such changes lead to hyperglycemia and intolerance to glucose.[10,13] The reduction in the function of b-cells and the mass of b-cells may be due to raised inflammation, lipotoxicity, and glucotoxicity.[13–15]
The expression of 1a-hydroxylase enzyme and vitamin D receptor transcript by the b-cells Islets of Langerhans promotes 25-hydroxycholecalciferol converting to 1,25-dihydroxycholecalciferol.[10] The vitamin D response element in the gene-promoting receptor region indicates that vitamin D has a considerable corollary in regulating insulin action [Figure 1].[16] Several studies have reported that in vivo, a deficiency in vitamin D resulted in a fall in insulin levels. There was also noted impairment of secretion of insulin by isolated islets.[17–19] Also, when vitamin supplements were introduced into mice with vitamin D deficiency, the insulin-secreting function of the islets was restored.[17,18,20–22] Such studies suggest that vitamin D directly affects the insulin-secreting function of pancreatic islets. A significant decrease in serum insulin levels and expression of Ins2 (gene providing instruction for insulin secretion) was observed in vitamin D receptor mutant mice.[10,21] Findings as such also indicate that the insulin expression-related gene involved in insulin expression and secreting is influenced by vitamin D and its receptor.
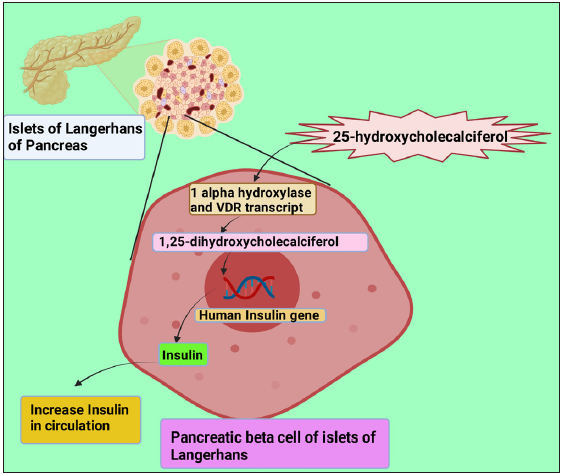
- Illustrates the conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol (the active form of vitamin D) by 1-a-hydroxylase and Vitamin D receptor transcript in the pancreatic b-cells of Islets of Langerhans. This active form of Vitamin D promotes the human insulin gene and increases the formation of insulin. VDR: Vitamin D Receptor. This figure has been drawn with the premium version of BioRender (https://biorender.com/accessed on 01 November 2023) with license number EF261KOSYA. Image credit: Rahnuma Ahmad
Clinical studies on T2DM, prediabetic, and non-diabetic subjects have noted the influence of vitamin D on b-cells of the islet of Langerhans in humans.[23–25] Despite evidence that connects vitamin D to the function of islets, it is not clear whether providing vitamin D treatment would improve insulin secretion based on several clinical trials.[26–28]
MECHANISMS AIDING IN THE REGULATION OF SYNTHESIS AND SECRETION OF INSULIN BY VITAMIN D
Several mechanisms help vitamin D in regulating the secretion and formation of insulin. One such mechanism is the binding of 1,25 dihydroxycholecalciferol with the vitamin D receptor, leading to gene expression for glucose transport, insulin secretion, and b cell growth.[16,29] There is also an influence of vitamin D on the concentration of Ca2+ within the cell, which may regulate the secretion of insulin indirectly. 1,25-dihydroxycholecalciferol causes b cell membrane depolarization, opening Ca2+ channels and increasing Ca2+ levels in the cell. Ca2+ promotes insulin vesicle mobilization and insulin release from b-cells by exocytosis.[30–33]
1,25-dihydroxycholecalciferol also causes activation of PKA and phosphorylation of Ca2+ channel-related protein and thus increases the activity of Ca2+channels.[32] Voltage-gated Ca2+ channels are also regulated through activating receptors for vitamin D. The active form of the vitamin increases insulin secretion.[34] Ca2+ release from the endoplasmic reticulum is promoted by vitamin D through augmenting PLC production and activating inositol triphosphate.[33,34] Vitamin D also helps to maintain Ca2+ concentration by regulating the expression of calbindin (a protein that binds Ca2+).[10,35,36]
ANTI-INFLAMMATORY AND STRESS FACTOR-REDUCING FUNCTION OF VITAMIN D
Vitamin D suppresses inflammation by directly suppressing the activation of the Nuclear Factor kB (NF-kB) transcription factor. Vitamin D also suppresses endoplasmic reticulum stress and Islet Amyloid polypeptide-mediated dysfunction of b-cells.[37] Vitamin D helps downregulate endoplasmic reticulum stress inducers from monocytes, islets, and liver like p-IREa, CHOP, and p-PERK.[38] This may be due to either direct repressing of gene expression for endoplasmic reticulum stress or effects secondary to the anti-inflammatory activity of vitamin D.[10]
Even though the protective effect of this vitamin on islets has been noted, supplementation with vitamin D has yet to clearly show improvement in glucose metabolism.1,39–42] Such findings may be attributed to a fall in the receptor of vitamin D expression in islets in the case of subjects with diabetes.[43] A study performed in mice noted that vitamin D receptor overexpression in the islets was needed to improve the dysfunction of the islet’s cells, indicating that vitamin D receptor activation above normal may be required to cause islets of Langerhans functional improvement.[43] Combining vitamin D and inhibitors of BRD9 can help simultaneous b-cells anti-inflammatory mechanisms activation and prevention of islet dysfunction, as has been observed in studies involving T2DM animal models.[37] Vitamin D also regulates non-endocrine cells and non-b endocrine cells of islets; for example, vitamin D receptors are expressed in macrophages of the islets, indicating that vitamin D influences the immune cells of the islet.[44]
SENSITIVITY OF INSULIN AND RESISTANCE AND VITAMIN D
In prediabetic and diabetic individuals, insulin’s ability to promote glucose entry into cells is impaired, also known as insulin resistance (IR). Regulation of insulin sensitivity for cells by vitamin D has been observed.[39] It has been observed in several studies that the active form of vitamin D promotes the expression of insulin receptors, which leads to raised sensitivity to insulin.[16,45,46] An association between vitamin D-activated proliferator-activated receptor (PPAR) d and increased insulin sensitivity has been found.[47,48] Studies recently have been performed to note the role of vitamin D in specific tissues concerning insulin sensitivity. Manna et al. found that GLUT-4 translocation in myotubes was promoted by 1,25-dihydroxycholecalciferol by activating Sirtuin 1 (SIRT1), Insulin receptor substrate-1 (IRS-1) phosphorylation and, therefore, enhancing uptake of glucose by skeletal muscle.[7] Zhou et al. suggested from their study that 1,25-dihydroxycholecalciferol reduced resistance to insulin myotube cells of skeletal muscle.[49] Also, translocation of GLUT-4 and rise in glucose uptake occurs when enhanced vitamin D activation promotes Ca2+ concentration in skeletal muscle [Figure 2].[50] Such studies indicate that vitamin D protects skeletal muscle against IR. In a study done with diabetic animal models, it was reported that a reduction in the expression of insulin receptor genes in the liver might be improved by using vitamin D.[51] However, another study did not find any effect of vitamin D on the transcription of the insulin receptor gene in mice’s liver on a high-fat diet.[52]
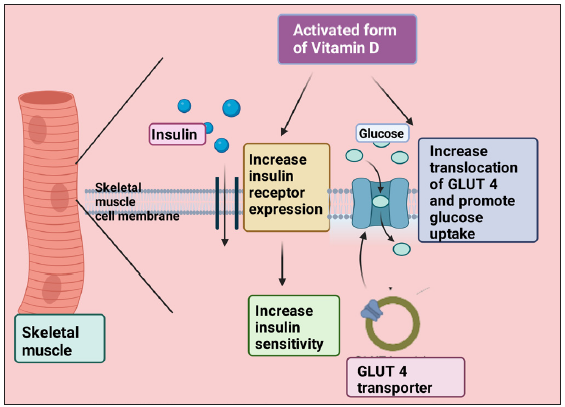
- Demonstrate the promotion of insulin receptor expression and translocation of GLUT 4 transporter by an activated form of vitamin D and thus increase insulin sensitivity and glucose uptake. GLUT: Glucose Transporter. This figure has been drawn with the premium version of BioRender (https://biorender.com/accessed on 01 November 2023) with license number LA261KYXZE. Image credit: Rahnuma Ahmad
A recent study on diet-induced obese experimental animals demonstrated the anti-inflammatory effect of vitamin D as they reported that activated vitamin D receptors acted on macrophages of the liver to lower inflammation in the liver, which in turn resulted in a decrease in IR.[53] Vitamin D causes downregulation of inflammatory cytokines like TNF-a, IL-1 b, and IL-6) and chemokines like CXCL10, CXCL11, and CCL5 from adipose cells and immune cells [54–57] and therefore decrease inflammation. A decrease in transcript and protein levels of TLR4 and TLR2 utilizing vitamin D receptors was suggested to be the possible mechanism for this downregulation of cytokines and chemokines by a study conducted on human monocytes.[58]
Vitamin D also suppresses MAPK signaling and NF-kB via vitamin D receptor to impart its anti-inflammatory activity.[59,60] Recruitment of monocytes is also inhibited by vitamin D and its receptor into adipocytes, and anti-inflammatory M2 macrophage is promoted in the adipose tissue.[61]
THE PLEIOTROPIC ROLE OF VITAMIN D AND VITAMIN D RECEPTOR IN RESISTANCE TO INSULIN
The pleiotropic role of vitamin D and its receptor in resistance to insulin may be through several mechanisms. There is induction of parathyroid hormone by vitamin D, which decreases resistance to insulin by raising the amount of GLUT-4 and GLUT-1 in muscle, liver, and adipose tissue deficient in vitamin D.[62,63] Renin-angiotensin-aldosterone system exhibits the ability to impair b cell activity. Vitamin D suppresses this system that, along with its b cell-impairing ability, also can cause hindrance of GLUT 4 recruitment.[64,65] The Ca2+/CaMKKb/AMPK pathway may be activated by high doses of vitamin D supplementation, which would reduce IR and endoplasmic reticulum stress.[66] Reactive oxygen species, an activating factor for IR formation, is prevented by vitamin D.[67]
The protective effect of 1,25-dihydroxycholecalciferol in resistance to insulin has been found in several studies. A study by Chiu et al. on individuals with normal glucose tolerance observed that subjects suffering from vitamin D deficiency had more risk of IR development.[68] Other research works noted that a low active form of vitamin D level in plasma was a factor for the risk of T2DM development.[69,70] Vitamin D supplementation has been shown to reduce IR and raise insulin secretion.[26,27,71] Another study on three T2DM subjects reported that supplementation with ergocalciferol improved IR.[10,72] However, a study performed on individuals with normal vitamin D found no improvement in glucose homeostasis on supplementation with vitamin D.[10,73] Such findings warrant further investigation on a broader scale to understand the effect of vitamin D on IR.
DEFICIENCY OF VITAMIN D AND TYPE 2 DIABETES MELLITUS
The association has been noted in previous studies between deficiency of vitamin D and dysfunction of islets, IR, and raised incidence of T2DM.[39] A cohort study conducted on 9841 subjects found a greater risk of T2DM in participants with low 25-hydroxycholecalciferol in plasma.[74] Several other studies have reported similar outcomes.[75–78] Song et al., in a meta-analysis which included four thousand nine hundred and ninety-six T2DM cases, observed a link between a lower risk of diabetes and higher plasma vitamin D levels. A 10 nmol/L rise in 25-hydroxycholecalciferol in plasma was associated with a 4% decrease in the incidence of T2DM.[79] A study was performed in California in which islet secretion capacity and index of insulin sensitivity were measured, and they found a positive association between 25-hydroxycholecalciferol level in plasma and function of b-cells and sensitivity of insulin.[80] While some studies have found a link between vitamin D deficiency and T2DM, others have not observed any significant association between these parameters.[81–83]
A randomized, double-blinded, and placebo-controlled clinical trial found a significant increase in sensitivity to insulin on supplementing with vitamin D3 for six months compared to the placebo.[24] Other trials done with vitamin D-deficient overweight subjects[84] and individuals with fasting blood glucose impairment[85] also noted similar findings. There was an improvement in fasting blood glucose and insulin levels after being supplemented with vitamin D[86] and HOMA-IR.[11,86] However, other trials with vitamin D supplementation did not reduce the risk of DM in subjects who were at high risk.[73,87] Thus, further research is required to understand whether vitamin D supplementation may prevent T2DM.
Genes involved in the insulin action and secretion pathway may be regulated directly. Vitamin D shows its anti-inflammatory effect by acting on the immune cells of tissue, decreasing systemic and local inflammation. This led to averting dysfunction of muscle, liver, and islets. In vitamin D deficient individuals, normalization of vitamin D has been found to lower the risk of T2DM. However, other clinical trials did not observe similar findings. Thus, the optimum level of vitamin D and whether supplementation with vitamin D has reversible and preventive effects for T2DM needs to be studied on a large scale.
Reference
- Serum vitamin D levels in relation to type-2 diabetes and prediabetes in adults: A systematic review and dose-response meta-analysis of epidemiologic studies. Crit Rev Food Sci Nutr. 2022;62:8178-98.
- [Google Scholar]
- History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;3:479.
- [Google Scholar]
- Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319-29.
- [Google Scholar]
- Serum 25-hydroxyvitamin D is a reliable indicator of vitamin D status. Am J Clin Nutr. 2011;94:619-20.
- [Google Scholar]
- Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;158:1-235.
- [Google Scholar]
- Role of vitamin D on the expression of glucose transporters in L6 myotubes. Indian J Endocrinol Metab. 2013;17:S326-8.
- [Google Scholar]
- Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high-fat diet-fed diabetic mice. Arch Biochem Biophys. 2017;615:22-34.
- [Google Scholar]
- Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS One. 2018;13:e0189707.
- [Google Scholar]
- Vitamin D3 intake as regulator of insulin-degrading enzyme and insulin receptor phosphorylation in diabetic rats. Biomed Pharmacother. 2017;85:155-9.
- [Google Scholar]
- Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients. 2023;15:1997.
- [Google Scholar]
- Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J Diabetes. 2015;6:1057-64.
- [Google Scholar]
- Nutrient-induced metabolic stress, adaptation, detoxification, and toxicity in the pancreatic b-cell. Diabetes. 2020;69:279-90.
- [Google Scholar]
- Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223-30.
- [Google Scholar]
- Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823-5.
- [Google Scholar]
- Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84-90.
- [Google Scholar]
- Vitamin D Increases glucose stimulated insulin secretion from insulin producing beta cells (INS1E) Int J Endocrinol Metab. 2019;17:e74255.
- [Google Scholar]
- Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta Endocrinol (Copenh). 1984;105:528-33.
- [Google Scholar]
- Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17:509-11.
- [Google Scholar]
- Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J Endocrinol. 1999;160:87-95.
- [Google Scholar]
- Effect of Vitamin D Supplementation on Glucose Control and Inflammatory Response in Type II Diabetes: A Double-Blind, Randomized Clinical Trial. Int J Endocrinol Metab. 2015;13:e22604.
- [Google Scholar]
- Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: A randomized, placebo-controlled trial. Eur J Endocrinol. 2019;181:287-99.
- [Google Scholar]
- Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33:1379-81.
- [Google Scholar]
- Effect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. 1986;1:187-92.
- [Google Scholar]
- The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57:258-61.
- [Google Scholar]
- Pancreatic secretion in man with subclinical vitamin D deficiency. Diabetologia. 1986;29:34-8.
- [Google Scholar]
- Unraveling the effects of 1,25OH2D3 on global gene expression in pancreatic islets. J Steroid Biochem Mol Biol. 2013;136:68-79.
- [Google Scholar]
- 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136:2852-61.
- [Google Scholar]
- Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev. 2003;55:105-31.
- [Google Scholar]
- Calcium signaling in pancreatic b-cells in health and in Type 2 diabetes. Cell Calcium. 2014;56:340-61.
- [Google Scholar]
- Vitamin D and pancreas: The role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr. 2017;57:3472-88.
- [Google Scholar]
- 1,25-Dihydroxyvitamin D3 enhances glucose-stimulated insulin secretion in mouse and human islets: A role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J Steroid Biochem Mol Biol. 2019;185:17-26.
- [Google Scholar]
- Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267:E356-60.
- [Google Scholar]
- Low vitamin D-modulated calcium-regulating proteins in psoriasis vulgaris plaques: S100A7 overexpression depends on joint involvement. Int J Mol Med. 2016;38:1083-92.
- [Google Scholar]
- Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287:38482-94.
- [Google Scholar]
- Vitamin D and diabetes: Where do we stand? Diabetes Res Clin Pract. 2015;108:201-9.
- [Google Scholar]
- The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017-29.
- [Google Scholar]
- Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31:701-7.
- [Google Scholar]
- Vitamin D supplementation and type 2 diabetes: A substudy of a randomized placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2009;38:606-9.
- [Google Scholar]
- Vitamin D Receptor Overexpression in b-Cells Ameliorates Diabetes in Mice. Diabetes. 2020;69:927-39.
- [Google Scholar]
- Expansion of Islet-Resident Macrophages Leads to Inflammation, Affecting b Cell Proliferation and Function in Obesity. Cell Metab. 2019;29:457-74.e5.
- [Google Scholar]
- Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383-91.
- [Google Scholar]
- Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3) Cell Biochem Funct. 2002;20:227-32.
- [Google Scholar]
- Vitamin D3 supplementation improves testicular function in diabetic rats through peroxisome proliferator-activated receptor-g/transforming growth factor-beta 1/nuclear factor-kappa B. J Diabetes Investig. 2019;10:261-71.
- [Google Scholar]
- Vitamin D increases PPARg expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017;36:54-9.
- [Google Scholar]
- 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab Res Rev. 2008;24:459-64.
- [Google Scholar]
- Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330-5.
- [Google Scholar]
- Effect of vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic rats. Br J Nutr. 2012;108:1410-8.
- [Google Scholar]
- Influence of vitamin D treatment on transcriptional regulation of insulin-sensitive genes. Metab Syndr Relat Disord. 2013;11:283-8.
- [Google Scholar]
- Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology. 2020;71:1559-74.
- [Google Scholar]
- Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol Nutr Food Res. 2012;56:1771-82.
- [Google Scholar]
- Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high-fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011;10:37.
- [Google Scholar]
- Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients. 2020;12:342.
- [Google Scholar]
- Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-30.
- [Google Scholar]
- Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361-70.
- [Google Scholar]
- 1,25-Dihydroxyvitamin D₃ suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kB activation. Arch Biochem Biophys. 2011;507:241-7.
- [Google Scholar]
- Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127-35.
- [Google Scholar]
- Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219-46.
- [Google Scholar]
- Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology. 1994;135:1837-44.
- [Google Scholar]
- The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int J Mol Sci. 2020;21:6644.
- [Google Scholar]
- Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin-angiotensin system in mice. Diabetologia. 2013;56:553-62.
- [Google Scholar]
- The crosstalk between insulin and renin-angiotensin-aldosterone signaling systems and its effect on glucose metabolism and diabetes prevention. Curr Vasc Pharmacol. 2008;6:301-12.
- [Google Scholar]
- The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients. 2016;8:147.
- [Google Scholar]
- Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567-75.
- [Google Scholar]
- Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820-5.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55:2173-82.
- [CrossRef] [PubMed] [Google Scholar]
- Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55:1668-78.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in non-diabetic adults. Diabetes Care. 2007;30:980-6.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med J. 1998;74:365-6.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance--a prospective randomized, double-blind study. Acta Med Scand. 1987;222:361-7.
- [CrossRef] [PubMed] [Google Scholar]
- Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and meta-analysis. Clin Chem. 2013;59:381-91.
- [CrossRef] [PubMed] [Google Scholar]
- Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813-8.
- [CrossRef] [PubMed] [Google Scholar]
- Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666-71.
- [CrossRef] [PubMed] [Google Scholar]
- Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990–2000. Diabetes. 2008;57:2619-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care. 2013;36:1422-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: The Tromsø study. Eur J Nutr. 2010;49:401-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D status in relation to glucose metabolism and type 2 diabetes in septuagenarians. Diabetes Care. 2011;34:1284-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lack of association between 25(OH)D levels and incident type 2 diabetes in older women. Diabetes Care. 2011;34(3):628-34.
- [Google Scholar]
- Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: The Hoorn study. Nutr Metab Cardiovasc Dis. 2012;22:883-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial. Am J Clin Nutr. 2014;100:152-60.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Transl Res. 2011;158:276-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol Metab Syndr. 2013;5:8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Response to Letter to the Editor from Dalan: “Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D?”. J Clin Endocrinol Metab. 2021;106:e1928-e1929.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





