Translate this page into:
Clinical profile and comorbidity patterns in subclinical thyroid disorders
* Corresponding author: Dr. Rahul Garg, MD Medicine, Department of Medicine, Farukh Husaain Medical College, Agra, India. gargrahul27@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garg R, Thakre A, Prakash P. Clinical profile and comorbidity patterns in subclinical thyroid disorders. J Health Sci Res. doi: 10.25259/JHSR_18_2025
Abstract
Objectives
Subclinical thyroid disorders present a significant challenge in clinical practice due to their subtle presentation and potential health impacts. Their prevalence and clinical manifestations in the North Indian population remain inadequately characterized. The objectives of this study were to analyze the clinical profile, biochemical parameters, and associated comorbidities in patients with subclinical hypothyroidism (SCH) and subclinical hyperthyroidism (SCHyper) at a North Indian tertiary care center.
Material and Methods
This cross-sectional observational study was conducted at a tertiary care hospital in North India from July 2024 to January 2025. Adult patients diagnosed with SCH and SCHyper based on thyroid function tests were included. Detailed clinical profiles, biochemical parameters, and associated comorbidities were analysed.
Results
Of 385 patients screened, 68 (17.7%) had SCH and 14 (3.6%) had SCHyper. The mean age was 46.8±12.4 years for SCH and 49.2±13.8 years for SCHyper patients. Female predominance was observed in both groups (SCH: 72.1%, SCHyper: 64.3%). Common symptoms in SCH included fatigue (51.5%), dry skin (69.1%), and cold intolerance (39.7%). SCHyper patients commonly presented with palpitations (42.9%), anxiety (35.7%), and heat intolerance (28.6%). Anti thyroid peroxidase (TPO) antibodies were positive in 45.6% of SCH and 28.6% of SCHyper patients. Significant associations were found between SCH and dyslipidemia (41.2%, p=0.008), obesity (32.4%, p=0.016), and metabolic syndrome (27.9%, p=0.023). SCHyper showed significant associations with anxiety disorders (28.6%, p=0.011), atrial fibrillation (21.4%, p=0.014), and osteoporosis (14.3%, p=0.032).
Conclusion
Despite being termed “subclinical,” these thyroid disorders present with distinct clinical features and significant comorbidities. The findings suggest that these conditions warrant careful clinical evaluation, particularly for cardiovascular and metabolic risk factors in SCH, and cardiac arrhythmias and bone health in SCHyper.
Keywords
Free thyroxine (FT4)
Free triiodothyronine (FT3)
Subclinical hypothyroidism (SCH)
Subclinical hyperthyroidism (SCHyper)
Thyroid-stimulating hormone (TSH)
INTRODUCTION
Subclinical thyroid disorders represent a state of mild thyroid dysfunction characterized by abnormal thyroid-stimulating hormone (TSH) levels, with normal free thyroxine (FT4) and free triiodothyronine (FT3) levels.[1] These conditions have gained significant attention due to their potential impact on various health outcomes and quality of life. The clinical significance of these disorders lies in their association with various cardiovascular, metabolic, and cognitive complications.
Subclinical hypothyroidism (SCH) is defined by elevated TSH with normal FT4 levels, while subclinical hyperthyroidism (SCHyper) is characterized by suppressed TSH with normal FT4 and FT3 levels.[2] The prevalence of these conditions varies significantly across different populations, with SCH being more common, affecting 3-16.9% of the general Indian population,[3-9] and SCHyper affecting around 0.2-11.3% of the global population[10] and around 1.6% of the Indian population.[3] The prevalence increases with age and is higher in women. Varying prevalence rates in some regions could be due to iodine deficiency and autoimmune thyroid disorders.[4] A major epidemiological study conducted in eight cities of India reported an overall prevalence of thyroid dysfunction of 10.95%, with SCH being the most common presentation.[3] However, comprehensive data on the clinical profile of patients with subclinical thyroid disorders from North India remains limited.
The clinical significance of subclinical thyroid disorders remains a subject of ongoing debate, particularly regarding the threshold for treatment initiation. Various studies have suggested associations between these conditions and adverse cardiovascular outcomes, cognitive decline, osteoporosis, metabolic disturbances and adverse pregnancy outcomes.[11-15] Understanding the clinical profile and associated comorbidities becomes crucial for appropriate management decisions.
The clinical significance of these subclinical disorders lies in their potential progression to overt thyroid dysfunction and the associated health risks. Early detection through screening can facilitate timely interventions, potentially preventing complications and improving overall health outcomes.[16] However, the absence of specific symptoms often leads to these conditions going undetected, highlighting the importance of routine screening, especially in high-risk populations.
This study aimed to analyze the clinical presentation, biochemical parameters, and associated comorbidities in patients with SCH and SCHyper at a tertiary care center in North India. The findings may help in better characterization of these disorders in the Indian population and guide management strategies.
MATERIAL AND METHODS
Study design and setting
This cross-sectional observational study was conducted at the Department of Medicine at a tertiary care hospital in North India, from July 2024 to January 2025. The study protocol was approved by the institutional ethics committee, and written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki. Participant confidentiality was maintained by assigning unique identification codes, and all personal identifiers were removed from the dataset before analysis. No financial compensation was provided to participants. To ensure inclusivity, the study accommodated non-English speakers by providing consent forms and conducting interviews in Hindi and other regional languages as needed, with trained translators available.
Study population
Adult patients (≥18 years) diagnosed with SCH (TSH >4.68 μIU/mL with normal FT4) or SCHyper (TSH <0.46 μIU/mL with normal FT4 and FT3) were included. Exclusion criteria included:
-
Pregnancy
-
Known thyroid disorders
-
Use of medications affecting thyroid function (amiodarone, lithium, glucocorticoids)
-
Severe systemic illnesses
-
Recent hospitalization
-
Major surgery within the past 3 months
-
Psychiatric illness
Data collection
Demographic details, clinical features, and medical history were recorded using a structured proforma. A detailed history, including symptoms, duration of illness, family history, and medication history, was obtained. Physical examination findings, including anthropometric measurements, vital signs, and systemic examination, were documented.
Laboratory investigations
Thyroid function tests were performed using chemiluminescent immunoassay. The reference ranges were: TSH: 0.46-4.68 μIU/mL, FT4: 12-30 pmol//L, FT3: 2.7-5.2 pg/mL. Additional investigations included a complete blood count, lipid profile, fasting blood glucose, anti-thyroid peroxidase (TPO) antibodies, electrocardiogram (in selected cases), and bone mineral density (in selected cases).
Statistical analysis
Data analysis was performed using SPSS version 25.0. Continuous variables were expressed as mean ± SD, and categorical variables as frequencies and percentages. Chi-square test and Student’s t-test were used for comparisons. Multivariate analysis was performed to identify independent associations. P-value <0.05 was considered statistically significant.
RESULTS
Demographic characteristics
Of 385 patients screened, 68 (17.7%) had SCH and 14 (3.6%) had SCHyper. The mean age was 46.8±12.4 and 49.2±13.8 years for SCH and SCHyper patients. Female predominance was observed in both groups (SCH: 72.1%, SCHyper: 64.3%), as shown in Table 1. The age distribution showed a peak in the 41-50 years age group for both conditions [Figure 1].
| Characteristic |
Subclinical hypothyroidism (SCH) |
Subclinical hyperthyroidism (SCHyper) |
|---|---|---|
| Number of patients | 68 | 14 |
| Females | 49 (72.1%) | 9 (64.3%) |
| Males | 19 (27.9%) | 5 (35.7%) |
| Mean age (in years) | 46.8±12.4 | 49.2±13.8 |
| Mean TSH (μIu/mL) | 7.3±1.9 | 0.24±0.11 |
| Mean FT3 (pg/mL) | 3.3±0.2 | 4.2±0.4 |
| Mean FT4 (pmol/L) | 15.91±1.9 | 21.2±1.9 |
| Anti-TPO Ab positive (%) | 45.6% | 28.6% |
TSH: Thyroid stimulating hormone, FT3: Free triiodothyronine, FT4: Free thyroxine, Anti-TPO Ab: Anti-thyroid peroxidase antibody
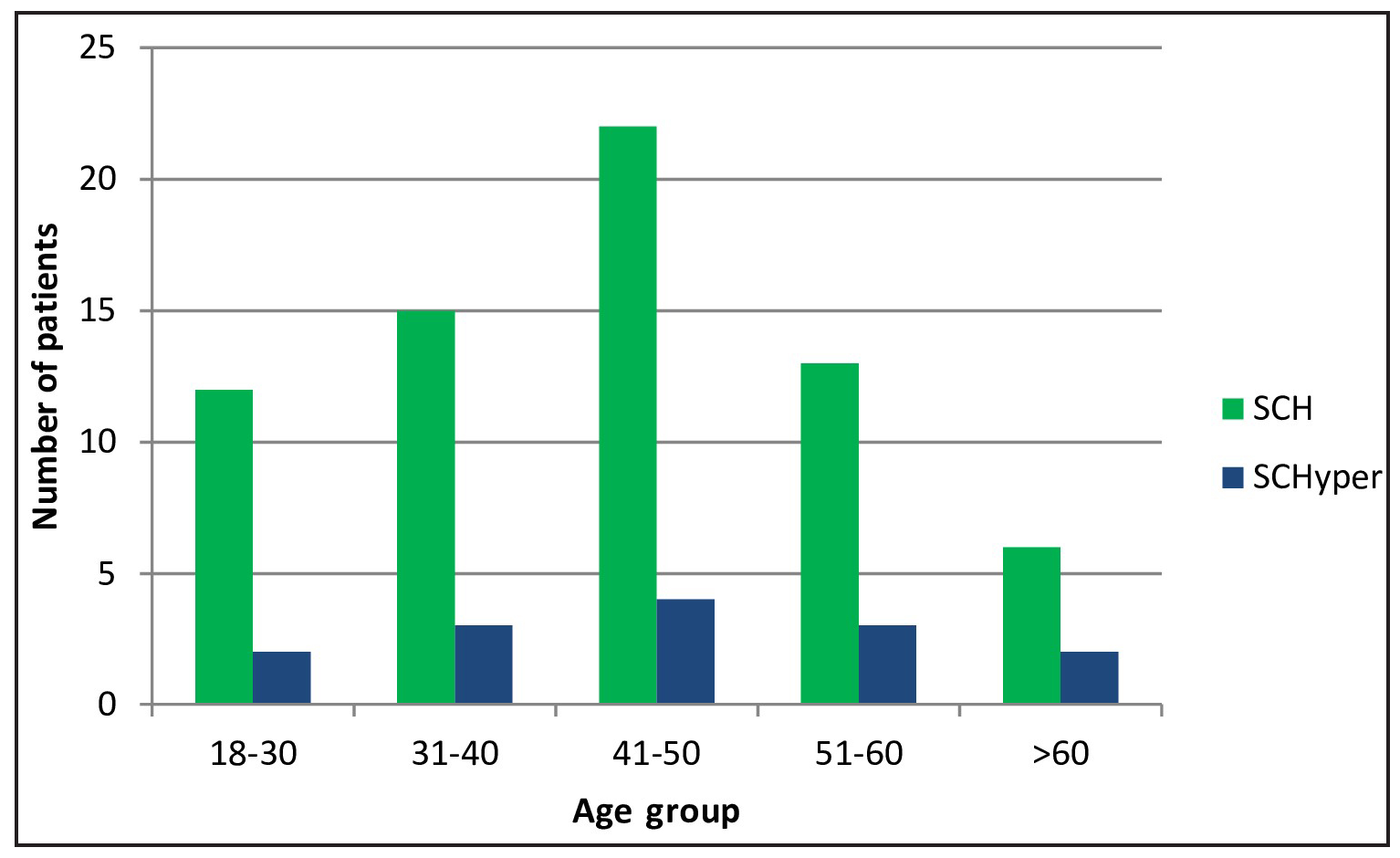
- Age distribution of subclinical thyroid disorders. SCH: Subclinical hypothyroidism, SCHyper: Subclinical hyperthyroidism.
Clinical features
In patients with SCH (n=68), the most common symptoms were dry skin (69.1%), fatigue (51.5%), and cold intolerance (39.7%). Other significant symptoms included constipation (29.4%), weight gain (23.5%), hair loss (17.6%), memory impairment (14.7%), and depression (11.8%), as shown in Figure 2. Among female patients with SCH, 30.6% reported menstrual irregularities. For SCHyper patients (n=14), the predominant symptoms were palpitations (42.9%), anxiety (35.7%), and heat intolerance (28.6%). Additional symptoms included weight loss and tremors (both 21.4%), sleep disturbances and muscle weakness (both 14.3%), as shown in Figure 3. Menstrual irregularities were reported by 22.2% of female patients with SCHyper.

- Clinical features in patients of subclinical hypothyroidism.

- Clinical features in patients of subclinical hyperthyroidism.
Biochemical parameters
Patients with SCH showed a mean TSH level of 7.3±1.9 μIU/mL (range: 4.6-12.4), mean FT4 of 15.91±1.9 pmol/L, and mean FT3 of 3.3±0.2 pg/mL. Anti-TPO antibodies were positive in 45.6% of SCH patients. Their lipid profile revealed a mean total cholesterol of 212±43 mg/dL and a mean LDL cholesterol of 136±35 mg/dL. In SCHyper patients, the mean TSH was 0.24±0.11 μIU/mL (range: 0.1-0.29), with mean FT4 of 21.2±1.9 pmol/L and mean FT3 of 4.2±0.4 pg/mL. Anti-TPO antibodies were positive in 28.6% of SCHyper patients [Table 1].
Associated comorbidities
Among patients with SCH, dyslipidemia was the most common comorbidity (41.2%, p=0.008), followed by obesity (BMI ≥30) affecting 32.4% (p=0.016), and metabolic syndrome present in 27.9% (p=0.023) of patients. Other comorbidities included hypertension (23.5%, p=0.062), type 2 diabetes (17.6%, p=0.093), and depression (11.8%, p=0.184), though these associations did not reach statistical significance [Table 2].
| Comorbidity | SCH (n=68) | Odds ratio (95%CI) | p-value |
|---|---|---|---|
| Major metabolic associations | |||
| Dyslipidemia | 28 (41.2%) | 2.4 (1.3-4.5) | 0.008* |
| Obesity (BMI≥30) | 22 (32.4%) | 2.1 (1.1-3.9) | 0.016* |
| Metabolic Syndrome | 19 (27.9%) | 2.0 (1.1-3.8) | 0.023* |
| Other cardiometabolic factors | |||
| Hypertension | 16 (23.5%) | 1.6 (0.9-3.1) | 0.062 |
| Type 2 diabetes | 12 (17.6%) | 1.7 (0.8-3.4) | 0.093 |
| Other comorbidities | |||
| Depression | 8 (11.8%) | 1.5 (0.7-3.5) | 0.184 |
| Anxiety disorder | 7 (10.3%) | 0.8 (0.3-1.9) | 0.428 |
| Osteoporosis | 5 (7.4%) | 1.1 (0.4-3.0) | 0.814 |
| Atrial fibrillation | 3 (4.4%) | 0.9 (0.2-3.1) | 0.872 |
| Female specific (n=49) | |||
| Menstrual irregularities | 15 (30.6%) | 1.8 (0.9-3.6) | 0.088 |
In SCHyper patients, anxiety disorders were observed in 28.6% (p=0.011), atrial fibrillation in 21.4% (p=0.014), and osteoporosis in 14.3% (p=0.032) of cases, all showing significant associations. Additional comorbidities included hypertension (21.4%, p=0.078) and type 2 diabetes (14.3%, p=0.212), but these associations were not statistically significant [Table 3].
| Comorbidity | SCHyper (n=14) |
Odds ratio (95% CI) |
p-value |
|---|---|---|---|
| Significant comorbidities | |||
| Anxiety disorder | 4 (28.6%) | 3.2 (1.2-8.7) | 0.011* |
| Atrial fibrillation | 3 (21.4%) | 3.9 (1.3-11.8) | 0.014* |
| Osteoporosis | 2 (14.3%) | 2.8 (1.1-7.4) | 0.032* |
| Cardiometabolic Factors | |||
| Hypertension | 3 (21.4%) | 1.4 (0.4-5.3) | 0.078 |
| Dyslipidemia | 3 (21.4%) | 0.9 (0.2-3.4) | 0.568 |
| Type 2 diabetes | 2 (14.3%) | 1.3 (0.3-6.2) | 0.212 |
| Obesity (BMI ≥30) | 2 (14.3%) | 0.7 (0.1-3.2) | 0.653 |
| Metabolic syndrome | 2 (14.3%) | 0.8 (0.2-3.6) | 0.784 |
| Other comorbidities | |||
| Depression | 1 (7.1%) | 0.8 (0.1-6.5) | 0.842 |
| Female-specific (n=9) | |||
| Menstrual irregularities | 2 (22.2%) | 1.2 (0.2-5.6) | 0.824 |
DISCUSSION
This comprehensive study of subclinical thyroid disorders provides significant insights into their clinical presentation and associated comorbidities in the North Indian population. The findings have important implications for screening and management strategies.
The demographic characteristics observed in our study align with previous research, showing a female predominance and higher prevalence in middle-aged adults.[11] This gender disparity may be attributed to autoimmune factors and hormonal influences, particularly estrogen’s role in modulating thyroid function.
The prevalence rates in our tertiary care setting, 17.7% for SCH and 3.6% for SCHyper, exceed those reported in general population studies across India (3-16.9% for SCH[3-9] and 1.6% for SCHyper[3]). This difference likely stems from referral bias, as symptomatic patients are more frequently directed to specialty centers. While our SCHyper prevalence exceeds Indian averages, it remains within global ranges (0.2-11.3%),[10] possibly reflecting regional variations in iodine intake or autoimmunity patterns.
The substantial symptom burden in our patients challenges the traditional “subclinical” designation. In SCH, the high prevalence of dry skin (69.1%), fatigue (51.5%), and cold intolerance (39.7%) suggests that even mild thyroid hormone deficiency significantly affects peripheral tissue metabolism. Comparing our findings with other Indian studies, we observed comparable symptom profiles but with some regional variations. For instance, Deshmukh et al.[4] reported slightly higher rates of fatigue (69.2%) in their Mumbai cohort but found a lower prevalence of dry skin (40%) and cold intolerance (33.8%) compared to our North Indian population.
Similarly, in SCHyper patients, the predominance of palpitations (42.9%), anxiety (35.7%), and heat intolerance (28.6%) indicates that seemingly minor thyroid hormone excess produces discernible clinical effects. The significant association between SCHyper and anxiety disorders (28.6%, p=0.011) underscores the need for psychological evaluation in these patients.
The comorbidity analysis reveals distinct patterns with important clinical implications. In SCH, we identified significant associations with dyslipidemia (41.2%, p=0.008), obesity (32.4%, p=0.016), and metabolic syndrome (27.9%, p=0.023). These findings support Zhao et al.’s[17] conclusion that even mild thyroid hormone deficiency can disrupt lipid metabolism and energy homeostasis, potentially accelerating atherosclerosis as described in the Rotterdam Study.[13] When compared with Indian data, our observed association between SCH and dyslipidemia (41.2%) is markedly lower than the 61.25% reported by Chowdhury et al.[9] in their cohort, suggesting potential regional variations in metabolic responses to thyroid dysfunction or differences in dietary and lifestyle factors.
For SCHyper, the significant associations with atrial fibrillation (21.4%, p=0.014) and osteoporosis (14.3%, p=0.032) reinforce the findings of Delitala[12] and Blum et al.[18] The atrial fibrillation link has particular clinical relevance given its implications for stroke risk and anticoagulation decisions. The osteoporosis association highlights the need for bone health monitoring, especially in postmenopausal women.
The high prevalence of anti-TPO antibodies in SCH (45.6%) suggests autoimmune etiology as a major underlying cause, consistent with previous Indian studies.[3,4] This finding aligns closely with the 47.6% positivity rate reported by Deshmukh et al.[4] in their Mumbai cohort, but is lower than the 65% reported by Chowdhury et al.,[9] possibly reflecting regional variations in autoimmune thyroid disease patterns. This finding has implications for disease progression and management decisions, particularly regarding the initiation of replacement therapy.
The cardiovascular associations observed in both conditions merit particular attention. While SCHyper is linked to arrhythmias (particularly atrial fibrillation), SCH is associated with metabolic risk factors for atherosclerosis. These distinct pathways suggest that subclinical thyroid disorders may contribute to cardiovascular risk through different mechanisms, supporting Cooper and Biondi’s[1] assertion that these conditions warrant careful clinical evaluation.
Importantly, the non-significant trends observed in hypertension (23.5%, p=0.062 in SCH; 21.4%, p=0.078 in SCHyper) and type 2 diabetes (17.6%, p=0.093 in SCH; 14.3%, p=0.212 in SCHyper) suggest potential associations that might reach significance in larger cohorts, highlighting areas for further investigation.
These findings have several clinical implications. First, they support a more proactive approach to patient evaluation, considering both biochemical parameters and clinical manifestations. Second, they suggest that targeted screening may be beneficial in high-risk populations, particularly middle-aged women. Third, they indicate that management decisions should consider the patient’s comorbidity profile, especially regarding cardiovascular and metabolic risk.
Limitations of the study
Several limitations should be considered when interpreting our results. The single-center design may limit the generalizability of our findings to other populations and settings. The relatively short duration of follow-up prevented us from assessing long-term outcomes and natural disease progression. Additionally, our study’s setting in a tertiary care center may have introduced referral bias, potentially overestimating the prevalence and severity of symptoms and comorbidities. The relatively small sample size, particularly for SCHyper (n=14), significantly limited our statistical power to detect associations. This is reflected in the wide confidence intervals observed for several associations, such as atrial fibrillation in SCHyper (OR 3.9, 95% CI 1.3-11.8), indicating low precision in these estimates. This small sample size for SCHyper reflects both the lower prevalence of this condition in the general population and recruitment challenges in our setting but is nonetheless a significant limitation of our study. The lack of long-term outcome data also limits our ability to make definitive conclusions about the prognostic implications of our findings.
CONCLUSION
This comprehensive study reveals that subclinical thyroid disorders manifest with distinct clinical features and significant comorbidity associations, challenging the “subclinical” terminology. The high prevalence of symptoms and associated conditions, particularly the link between SCH and metabolic disorders, and SCHyper with cardiovascular complications, emphasizes the need for systematic screening and monitoring in high-risk populations. The findings support a more proactive approach to patient evaluation and management, considering both biochemical parameters and clinical manifestations. Future research should focus on long-term outcomes and optimal treatment strategies through multicenter studies with larger cohorts.
Ethical approval
The research/study approved by the Institutional Review Board at F H Medical College, Agra, number FHMC/IEC/R.Cell/2024/39, dated 11th July 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Update on subclinical thyroid dysfunction. Endocr J. 2022;69:725-38.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid disorders in India: An epidemiological perspective. Indian J Endocrinol Metab. 2011;15:S78-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence, clinical and biochemical profile of subclinical hypothyroidism in normal population in Mumbai. Indian J Endocrinol Metab. 2013;17:454-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Study on thyroid dysfunction in patients attending Rama Medical College Hospital & Research Centre: A hospital based study. J Med Sci Clin Res. 2015;3:7750-56.
- [Google Scholar]
- Prevalence of subclinical hypothyroidism in general adult population of northern India. J Med Sci Clin Res. 2020;8:595-97.
- [CrossRef] [Google Scholar]
- Hospital-based prevalence study on thyroid disorders in the Malwa region of Central India. Int J Curr Microbiol App Sci. 2015;4:604-11.
- [Google Scholar]
- Prevalence of overt and subclinical thyroid disease in patients tested for thyroid profile in CIMS Bilaspur (C.G.) Inter Jour of Clin Bio and Res. 2016;3:343.
- [CrossRef] [Google Scholar]
- A study correlating the effects of subclinical hypothyroidism on the known modifiable risk factors of coronary artery disease in Indian adults. J Assoc Physicians India. 2024;72:44-8.
- [CrossRef] [PubMed] [Google Scholar]
- Subclinical thyrotoxicosis: Prevalence, causes and choice of therapy. Eur J Endocrinol. 2017;176:R325-37.
- [CrossRef] [PubMed] [Google Scholar]
- Graves’ disease, multinodular goiter and subclinical hyperthyroidism. Ann Endocrinol (Paris). 2019;80:240-49.
- [CrossRef] [PubMed] [Google Scholar]
- Subclinical hyperthyroidism and the cardiovascular disease. Horm Metab Res. 2017;49:723-31.
- [CrossRef] [PubMed] [Google Scholar]
- Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: The Rotterdam study. Ann Intern Med. 2000;132:270-8.
- [CrossRef] [PubMed] [Google Scholar]
- Thyrotropin, hyperthyroidism, and bone mass. J Clin Endocrinol Metab. 2021;106:e4809-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical review: The thyroid in mind: Cognitive function and low thyrotropin in older people. J Clin Endocrinol Metab. 2012;97:3438-49.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Subclinical hypothyroidism. In: Stat Pearls. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536970/ [Last accessed 2025 Jan18]
- [Google Scholar]
- Subclinical hypothyroidism might worsen the effects of aging on serum lipid profiles. Thyroid. 2015;25:485-93.
- [CrossRef] [PubMed] [Google Scholar]
- Subclinical thyroid dysfunction and fracture risk. JAMA. 2015;313:2055.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






