Translate this page into:
Urine albumin and serum cystatin C in HIV-infected children on antiretroviral drugs: A cross-sectional hospital based study
*Corresponding author: Dr. Sylvester Sunday Edward, Department of Paediatrics, Obafemi Awolowo University Teaching Hospital, Opa Road, Ile-Ife, Nigeria. sylvesteredwardsunday@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Edward SS, Akomolafe RO, Adejuyigbe EA, Olukiran OS. Urine albumin and serum cystatin C in HIV-infected children on antiretroviral drugs: A cross-sectional hospital based study. J Health Sci Res. 2025;10:9-17. doi: 10.25259/JHSR_54_2024
Abstract
Objectives
Kidney diseases are known complications of human immunodeficiency (HIV) despite administration of antiretroviral drugs. There is limited information on early markers of renal glomerular functions among Nigerian children on highly active antiretroviral therapy (HAART). Urine albumin and serum cystatin C are sensitive indicators of renal dysfunction, and their timely detection could potentially prevent the progression of overt chronic kidney disease in this vulnerable population.To assess the renal glomerular functions using urine albumin–creatinine ratio and blood level of cystatin C in children living with HIV on HAART.
Material and Methods
This descriptive cross-sectional study involved 100 participants; the case group comprised 50 confirmed HIV-infected children, while the control participants were age- and sex-matched 50 HIV-negative children. The data analysis was done using the statistical packages for Social Science version 20.0
Results
The mean level of serum cystatin C among the HIV-positive subjects (0.57 ± 0.11 mg/L) was not statistically significant (P = 0.332) relative to the control (0.59 ± 0.11 mg/L). None of the HIV-infected and control subjects had abnormal concentrations of cystatin C nor its estimated glomerular filtration rate. Microalbuminuria was found in 16.0 and 8.0% of the recruited HIV-infected and control subjects, respectively. Only prolonged duration of HIV/AIDS was significantly associated with microalbuminuria, while other parameters such as World Health Organization (WHO) clinical staging, cluster of differentiation (CD)-4 cell count, and classes of HAART were not predictive of albuminuria.
Conclusion
The study showed that albuminuria was high among HIV-infected children on HAART, despite their normal serum levels of cystatin C and estimated glomerular filtration rate (GFR). A prolonged duration of HIV/AIDS was associated with the occurrence of microalbuminuria, which could be an early indicator of subclinical kidney impairment among HIV-infected subjects. The findings of this study indicate a high risk of cardiorenal complications among HIV-infected children on HAART. Consequently, regular renal and cardiovascular assessments are necessary for this vulnerable population to mitigate potential morbidity and mortality. However, further research is warranted to elucidate the pathogenesis, progression, and long-term outcomes of individuals with this finding.
Keywords
Children
Cystatin C
HIV
Kidney
Urine albumin
INTRODUCTION
Globally, about 38.4 million people have human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) and around 65,000 of them died from the disease in 2021.[1] HIV/AIDS may be complicated by kidney diseases in about 30% of patients.[2,3] Multifactorial factors, such as the HIV infection itself, coinfections, comorbidities, and their treatments, can contribute to renal diseases in patients living with the virus.[4] The most frequent finding on renal biopsy in Black patients with HIV infection is HIV-associated nephropathy (HIVAN).[5]
The initiation of highly active antiretroviral therapy (HAART) has resulted in the long-term survival of children living with HIV/AIDS and its multisystemic complications.[6] Diverse prevalence reports have been observed in studies on renal impairment among African HIV patients receiving HAART. According to Franey et al.,[7] the prevalence rates of moderate and severe renal impairment among HIV-positive patients in South Africa were 1.3.1 and 1.3%, respectively. Also, 11.5% of patients receiving antiretroviral therapy (ART) in Western Kenya were reported to have renal insufficiency in a study by Wools-Kaloustian et al.[8] Ayokunle et al.[9] in a study conducted in Nigeria observed that 47.6% of HIV/AIDS patients on ART had chronic kidney disease (CKD).
Urine albumin–creatinine ratio (UACR) and cystatin C significantly improve the risk prediction of kidney disease, heart failure, and mortality.[10] In a study by Choi et al.,[11] HIV-positive patients on HAART with cystatin C-based estimated glomerular filtration rate (eGFR-cys) of less than 60 mL/min/1.73 m2 and albuminuria had a nearly two-fold increase in mortality. Cystatin C is a better predictor of end-stage renal diseases (ESRD) than serum creatinine and modified glomerular filtration rate (GFR).[12,13] It is a useful marker of glomerular filtration rate, particularly in early renal impairment.[14,15] Urine albumin–creatinine ratio is used to assess microalbuminuria (now called albuminuria) or macroalbuminuria.[16] Albuminuria is an independent marker for chronic renal impairment and cardiovascular disease because it connotes increased endothelial permeability. It has been reported to be a precursor of HIV-associated kidney disease, which, if detected early and coupled with requisite intervention may slow the progression of the disease.[17]
Most of the evaluative studies on the assessment of serum cystatin C level and urine albumin–creatinine ratio among HIV-infected populations on HAART regimens were done in adults. Few studies that have been reported in HIV-positive children were carried out among those that were HAART-naïve. Long-term disease and drug toxicity assessments are important in children, who tend to take HAART for a longer duration than adults, and this may adversely affect their growth and development.[18] Presently, glomerular function using the early renal markers among Nigerian children on HAART for HIV/AIDS has not been extensively studied; hence, this study aims to assess the renal glomerular functions using urine albumin–creatinine ratio and blood cystatin C in HIV-positive children on HAART.
MATERIAL AND METHODS
The study was done at the Obafemi Awolowo University Teaching Hospitals Complex in Ile-Ife, Osun State. The institution has subunits, which include the Ife Hospital Unit (IHU) and the Comprehensive Health Center (CHC) in Ile-Ife. The virology clinic, located in IHU, is staffed with doctors, nurses, and other health personnel; it opens daily for pediatric and adult clinical consultation for retroviral disease patients. The outpatient section of IHU is involved in the management of patients who do not need admission; the staff are saddled with triaging patients with severe illnesses for prompt admission. The CHC is about 5 km from IHU. The unit provides outpatient services and also admits a few patients for observation.
Ethical approval was obtained from the “Ethics and Research Committee” of the health institution (ERC/2021/06/14). A total of 100 participants were recruited; the case group consisted of 50 confirmed and registered HIV-infected children who were receiving care at the Virology Research Clinic of Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife. The control group comprised age- and sex-matched 50 confirmed HIV-negative children who were either attending pediatric outpatient clinics in the hospital or accompanying patients’ relatives or siblings.
The recruited HIV-infected subjects were children aged 5–17 years who were attending the HIV clinic at OAUTHC and had been on antiretroviral drugs for at least 6 months. In addition, the researcher obtained caregivers’ informed consent and assent from children who were over 7 years. The exclusion criteria included the presence of overt features of kidney diseases (such as edema, bloody urination, and reduced urine volume) and a history of morbidities such as hypertension, chronic kidney diseases, or diabetes before the diagnosis of HIV/AIDS. Also, patients who had goitre or overt features of thyroid dysfunction and those with a history suggestive of urinary tract infection (frequent/urgent/painful urination) were excluded. The control subjects, who were sex- and age-matched HIV-negative children with similar inclusion and exclusion criteria as the HIV-positive group, were recruited after excluding HIV by the negative result using “Determine” antibody-based rapid test kit.
The weight of each participant was measured to the nearest 0.1 kg using an appropriate weighing scale. Similarly, the height of each participant was measured to the nearest 0.1 cm using a standard stadiometer. The clinical staging of HIV in the cases was done by the researcher according to the WHO criteria. Three milliliters (3 mL) of clean-catch midstream spot urine sample was obtained into a 5 mL plain bottle from each HIV-infected and control subject for estimation of urine albumin and creatinine levels. Two (2 mL) blood samples were obtained into each ethylenediaminetetraacetic acid (EDTA) bottle and plain bottle for CD4 estimation and serum cystatin C estimation, respectively.
Urine albumin was assayed using fluorescence immunoassay on ichroma™ tests reader (INS-MA-EN Rev.14, Boditech Med Incorporated, Republic of Korea), while urine creatinine was determined by automated spectrophotometry using Jaffe-kinetic method (Randox Human Multisera, HN1530-HE1532, Randox Company, United Kingdom). Urine albumin–creatinine ratio that was less than 30, 30–300, or >300 mg/g was considered normal, microalbuminuria, and macroalbuminuria, respectively, in line with the classification by Kidney Diseases Improving Global Outcome (KDIGO).[19]
The analysis of CD4 counts was done using a flow cytometer produced by Partec in Germany. The CD4 values greater than 500, 350–499, 200–349, or less than 200 cells per mm3 were categorized as insignificant, mild, advanced, or severe immunodeficiency, respectively. Serum cystatin C was analyzed using fluorescence immunoassay on an ichroma™ tests reader (INS-CC-EN Rev.03, Boditech Med Incorporated, Republic of Korea). In line with previous reports, serum cystatin C level above 1.0 mg/L was considered high.[20,21] The GFR (mL/min/1.73 m2) was estimated by the cystatin C-based equation derived by “Filler and Lepage” Log (GFR) = 1.962 + [1.123 × log (1/cystatin C)].[22]
Data analysis was done using the Statistical Package for Social Sciences (SPSS) version 20.0. Shapiro-Wilk test was used to assess the normality of the variables. Indices such as age and cystatin C, which were parametric, were expressed as mean ± standard deviation (SD), while the other nonparametric quantitative indices were expressed as median ± interquartile range (IQR). Comparison of means was determined by Student’s t-test and analysis of variance (ANOVA) for continuous parametric variables, while Mann Whitney-u test and Kruskal Wallis test were used for variables that were not normally distributed. The association between categorical variables was determined using Fisher’s exact test or Chi-square. P-value < 0.05 was considered statistically significant.
RESULTS
The ages of the study groups ranged between between 5 and 17 years, with a mean ± SD of 11.08 ± 3.40 years. There was no significant age difference between the groups because of appropriate matching (t(0.000, 98); P = 1.000). Twenty-eight (56.0%) participants in each of the sex-matched study groups were males, while 22 were females with a male-to-female ratio of 1.3:1 (P = 1.000) [Table 1].
| Variables | HIV-infected (50) | Non-HIV infected (50) | P-value |
|---|---|---|---|
| Age (years) | 11.08 ±3.40 | 11.08 ± 3.40 | 1.000a |
| Gender | |||
| Male | 28 (56.0) | 28 (56.0) | 1.000b |
| Female | 22 (44.0) | 22 (44.0) | |
| BMI (WHO classification) | |||
| Normal | 41 (82.0) | 43 (86.0) | 0.700b |
| Thin | 7 (14.0) | 4 (8.0) | |
| Very thin | 2 (4.0) | 3 (6.0) | |
| Cystatin C (mg/L) | 0.57 ± 0.11 | 0.59 ± 0.11 | 0.332a |
| e-GFRcyst (ml/min/1.732) | 164.15 ± 58.93 | 146.05 ± 27.12 | 0.500c |
| Urine albumin (mg/L) | 12.46 (5.10- 25.58)¥ | 5.25 (2.09-11.44)¥ | 0.001*c |
| Urine creatinine (µmol/L) | 6325.00 (3624.75-8595.25)¥ | 4742.00 ± 2881.25-7817.75¥ | 0.090c |
| UACR (mg/g) | 16.66 (9.05-28.54)¥ | 8.90 (5.58-15.64)¥ | 0.001*c |
a Student’s t-test; b Pearson chi-square; c Mann-Whitney U test; *Significant at P < 0.05. ¥Median with Interquartile range. BMI: Body mass index; e-GFRcyst: estimated glomerular filtration rate using cystatin-c; UACR: Urine albumin-creatinine ratio, WHO: World health organization.
A total of 82 and 86% of the HIV-infected subjects and control group, respectively, had normal body mass index (BMI) after plotting the absolute values against the WHO age and sex z-score charts. Although the absolute BMI of the control group was higher compared to the cases, there was no significant difference between them (U = 1021; P = 0.112). 45 (90.0%) HIV-infected subjects had normal height, while the remaining 10.0% were stunted. Only 1 (2.0%) participant in the control group was stunted. There was no significant difference in the height of the two groups (t(−1.081, 98); P = 0.282), as shown in the Table 1.
Biochemical indices of renal function in cases and control subjects
The HIV-infected subjects had a mean cystatin C concentration (0.57 ± 0.11 mg/L) that was not significantly different (t(−0.975, 98); P = 0.332) from the control subjects (0.59 ± 0.11). None of the HIV-infected and control subjects had abnormal cystatin C concentrations. Similarly, the cystatin C-based estimated GFR for the cases and the control subjects was not significantly different as shown in Table 1.
The median (IQR) urine albumin (mg/L) of the control subjects, 5.25 (2.09–11.44), was significantly lower (U = 760; P = 0.001) compared to the HIV-infected subjects, which was 12.46 (5.10–25.58). This was similar to the urine albumin to creatinine (mg/g), which was significantly lower (U = 772; P = 0.001) in the control subjects, [8.90 (5.58 –15.64)] relative to the cases [16.66 (9.05–28.54)].
Clinical and laboratory indices of HIV-infected subjects
Sixteen (32.0%) subjects had been diagnosed with HIV for over 10 years, and a similar number were diagnosed within the last 5–10 years, while 18 (36.0%) subjects were confirmed HIV-positive within the last 5 years [Figure 1]. From the time of diagnosis of HIV in the cases, the mean duration of the disease in the study population was 7.78 ± 4.06 years. Sixteen (32.0%) HIV-infected subjects were taking tenofovir disoproxil fumarate (TDF)-based HAART, while 23 (46.0%) were receiving lopinavir-based HAART. The remaining 11 were on HAART that neither contained TDF nor lopinavir as shown in Figure 2.
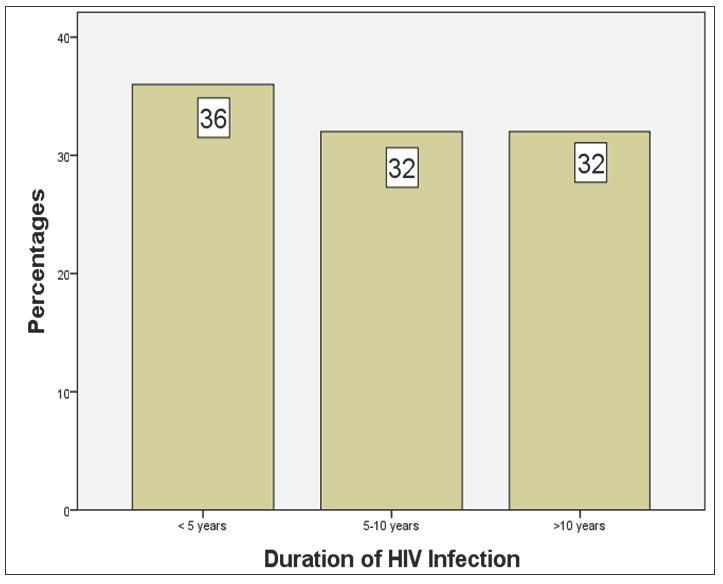
- Duration of HIV infection from the time of diagnosis. HIV: Human immunodeficiency virus

- Classes of highly active antiretroviral therapy in HIV-infected subjects. TDF: Tenofovir disoproxil fumarate
The median (IQR) CD4-cell count in the subjects was 917.50 (676.00–1239.00). Approximately 94% of the cases had CD4-cell counts ≥ 500 cells/mm3, which implied an insignificant immunological suppression. Only three subjects had mild to advanced immunological suppression.48 subjects (96.0%) had WHO Stage 1 disease while the remaining others were in Stage 2 at the time of recruitment. None of the subjects had advanced disease (Stage 3 or 4).
Comparison of variables of HIV-infected subjects and classification of urine albumin–creatinine ratio
There was no statistical difference in the classes of urine albumin–creatinine ratio across the WHO clinical staging of the patients (P = 0.720). The duration (years) of HIV in subjects with microalbuminuria and macroalbuminuria (11.00 ± 3.00 and 11.50 ± 4.75, respectively) were significantly higher (P = 0.033) compared with those who had normal urine albumin–creatinine ratio (6.50 ± 5.50). However, the CD4-cell count (cells/mm3) in participants with normal urine albumin-creatinine (UAC) (892 ± 648.25) relative to those with microalbuminuria (1049.50 ± 340.25) and macroalbuminuria (77550 ± 50) was also not significantly different (P = 0.940) as shown in Table 2.
| Variables | Urine albumin-creatinine | P-value | ||
|---|---|---|---|---|
| Normal | Microalbuminuria | Macroalbuminuria | ||
| WHO Staging: | ||||
| I (%) | 36 (94.7) | 8 (100.0) | 4 (100.0) | 0.720a |
| II (%) | 2 (5.3) | 0 (0.0) | 0 (0.0) | |
| Duration of | 6.50# ± 5.50 | 11.00# ± 3.00 | 11.50 ± 4.75 | 0.033*b |
| Disease (years) | ||||
| CD4-cell counts | 892.00 | 1049.50 | 775.00 | 0.94b |
| (cells/mm3) | (659.00-1307.25) | (768.75-1109.00) | (603.50-1377.00) | |
a Pearson chi-square χ2; bKrukas Wallis test’s t-test; # significantly different between-group (post hoc, 0.04); *significant at P < 0.05. WHO: World Health Organization; CD4: Cluster of differentiation, HIV: Human immunodeficiency virus.
Relationship between classes of antiretroviral drugs with urine albumin–creatinine ratio and estimated glomerular filtration rate
Although the median (IQR) urine albumin–creatinine ratio of HIV-treated subjects on TDF-based drugs was higher (19.06 [18.20–54.40]) than the values obtained for lopinavir-based (16.20 [9.16–26.39]) and those on other drugs (16.85 [10.53–22.93]), there was no statistical difference between the two groups (P= 0.920) [Table 3].
| Laboratory indices | HAART classes | P-value | ||
|---|---|---|---|---|
| TDF-based | LOP-based | NON-TDF/LOP | ||
| UACR (mg/g) |
19.05 (8.20-54.40)¥ |
16.20 (9.16-26.39)¥ |
16.85 (10.40-22.93)¥ |
0.920a |
| Cystatin C (mg/L) | 0.56 ± 0.10 | 0.57 ± 0.11 | 0.59 ± 0.10 | 0.820b |
| e-GFR (ml/min/1.73) | 170.70 ± 66.51 | 168.91 ± 59.98 | 153.93 ± 65.26 | 0.650a |
aKruskal Wallis test’s t-test; bANOVA; *significant at P < 0.05. ¥Median/Interquartile range. e-GFRCyst: estimated glomerular filtration rate using cystatin-c; UACR: Urine albumin-creatinine ratio; TDF: Tenofovir disoproxil fumarate, LOP: Lopinavir, HAART: Highly active antiretroviral therapy.
However, the mean cystatin C level among TDF-treated subjects was slightly lower (0.56 ± 0.10) compared with the values in subjects on lopinavir-based (0.57 ± 0.11) and non-TDF/lopinavir-based drugs (0.59 ± 0.10) but this was not statistically significant (P = 0.820). Similarly, there was no significant difference (P = 0.650) between the estimated glomerular filtration rates among subjects who were taking TDF compared with other drugs.
DISCUSSION
The study showed that microalbuminuria and macroalbuminuria are common among HIV-positive children in Ile-Ife, Osun State. Microalbuminuria was observed in 16.0% of the cases, which was comparably higher than 8.0% in the control subjects, who also had a lower prevalence of macroalbuminuria than the former. The median value of urine albumin–creatinine ratio was significantly higher in the cases compared with the control subjects.
In line with the prevalence of microalbuminuria in this study, Dimock et al.[23] found a prevalence rate of 15 % among HIV-positive youth and children in the United States of America. The finding was also closely similar to the report by Okechukwu et al.,[24] who found a microalbuminuria prevalence of 13.7% and proteinuria prevalence of 6.8% among the population of HAART-treated children in Abuja, Nigeria.
The prevalence rate of microalbuminuria in the present study was, however lower than 20.4% as obtained by Fredrick et al.[25] in Tanzania, 25.0% from a study in South Africa,[26] 28.8% from another Tanzanian study,[27] and 35.6% among HIV-positive adults in Nigeria.[28] It was, however much higher than 0% recorded by Bertilla et al.[29] in Enugu, Nigeria, 6.7% by Mudi et al.[17] in Kano, Nigeria as well as 12.0% that was earlier reported among HIV-positive children in another study by Eke et al.[30] in Port-Harcourt, Nigeria.
The variation in the prevalence of microalbuminuria among HIV-positive children within and outside Nigeria may be related to the diverse cutoff values that were used for defining microalbuminuria in various studies, coupled with various methods used in assaying urine albumin–creatinine.[27,29] The differences in the sample size and different study populations, as well as selection criteria could also account for the observed differences.[17,24,27] In addition, variations in the immunological status of recruited subjects and the use of HAART-naïve or HAART-treated subjects could also contribute to the differences.[17,26,30] HIV-positive patients with advanced AIDS stage, low CD4 count, high viral load, and severe malnutrition have been found to have a high likelihood of having microalbuminuria or proteinuria.[24,25,30,31] Most of the subjects in this study had normal immunological status and were predominantly well nourished.
Other indices such as environmental and genetic factors may also play a significant role in the variation of prevalence values in different studies by Ibadin et al. and Okpere et al.[32,33] Patients with factors such as hypertension, family history of hypertension and diabetes mellitus, puberty, and sickle cell anemia have been found to have a higher prevalence of microalbuminuria relative to those without risk factors as reported in previous studies.[32,33] Genetic factors are not frequently considered in many studies even though these intrinsic predispositions can also explain the occurrence of kidney diseases[34-37] in HAART-treated or HAART-naïve subjects, although these were not assessed in this study.
Microalbuminuria has been reported to correlate with the Framingham risk score and represents an important predictor for cardiovascular disease in subjects with a Framingham risk score <20%.[38] It presents in the urine early and has been reported to predict proteinuria in HIV-infected patients.[17,39] Clinically, none of the cases and control subjects with microalbuminuria in this study had any obvious features of kidney disease. This supports the finding that the majority of HIV-infected children with renal diseases had subclinical microalbuminuria,[9,40,41] which is an early pathologic marker of glomerular impairment.[17,42,43] The kidney is one of the key reservoirs of HIV, hence the virus can exert a direct pathogenic activity on it, with attendant renal glomerular or tubular abnormalities.[44]
It was observed that urine albumin–creatinine-based renal function showed no significant correlation with WHO stages of HIV disease in the age groups; there was also no association between immunological status, WHO clinical stage, and the incidence of albuminuria. This is consistent with the report that renal impairment was independent of many parameters for the clinical staging of HIV infection.[8] Also, renal impairment may be the first manifestation of HIV/AIDS in some patients.[21] In a large series by Winston et al.,[45] the authors found that about 50% of patients who were initially asymptomatic at presentation were later confirmed to have severe immunosuppression and HIVAN. However, there was a statistically significant difference between the duration of HIV/AIDS and albuminuria in this study; this is possibly due to the prolonged inflammatory impact of the disease on the kidneys coupled with the possible effects of drug mediators.[46-48]
As found in this study, the administration of HAART may not necessarily influence the occurrence of microalbuminuria as reported in previous studies.[17,27,49] HIV can still induce kidney damage despite suppressed viral load after the commencement of HAART because the kidneys may potentially serve as a reservoir for the virus.[44]
The study showed a remarkably significant difference between the median values of urine albumin as well as urine albumin-creatinine ratios among the HIV-infected subject relative to the control group. This may be due to the chronic impact and complications of HIV/AIDS coupled with the initiation of HAART, which has led to prolonged survival of children infected with HIV and its multisystemic morbidities.[4,6]
Although the introduction of HAART is known to reduce the burden and death in HIV-positive children,[50,51] long-term exposure to these drugs is potentially nephrotoxic.[41] Although isolated cases of renal toxicity have also been documented in almost all HAART, it has been recognized that specific antiretroviral agents, namely tenofovir disoproxil fumarate, atazanavir, and indinavir, possess established links with direct nephrotoxicity that are supported by some cohort of case studies.[46-48] Antiretroviral therapy has been reported to contribute to renal toxicity either by inducing nephropathy through crystal-deposit nephropathy and acute tubular necrosis or indirectly through pharmacologic interactions.[52] Renal toxicity and its long-term complications could increase in Nigerian HIV-positive children due to improved access to ART and anticipated longer survival.[41]
The present study showed zero prevalence of elevated serum cystatin C in the experimental population as well as the control group. As such, all the subjects had normal estimated glomerular filtration rates using cystatin C (eGFR-cys). This finding is at variance with the outcome of the study by Abiodun et al.,[21] who got a high prevalence of chronic kidney disease (10.7%) using a cystatin C-based approach among HIV-positive children in Benin City, Nigeria.Also, Esezobor et al.[20] found the prevalence of elevated cystatin C (≥1 mg/L) to be 16.7% among children with HIV infection in Lagos, Nigeria. The finding of this study is also dissimilar to the cystatin-based eGFR prevalence of CKD (15.2%) that was quoted by Jones et al.[53] in a study among 250 HIV-positive adults in Massachusetts and Rhode Island in the USA.
The reasons for the observed differences could be because the studies by Abiodun et al. and Esezobor et al.[20,21] were conducted in HAART-naïve subjects. As such, the inflammatory impacts of HIV infection on the kidneys had not been partly mitigated by antiretroviral drugs, hence their abnormal cystatin C findings. The reported high prevalence of chronic kidney disease (CKD) that was observed by Jones et al. (2008) in the United States could also be attributed to the longer duration of HIV disease because the study was done in the adult population for which some of the recruited participants had comorbidities such as viral hepatitis as well as diabetes mellitus. These comorbid conditions are known risk factors for the high prevalence of CKD in the general population.[53,54]
The finding of normal e-GFR in all the subjects is similar to the study by Ayokunle et al.,[9] who reported that a higher number of HIV-infected patients had normal or elevated GFR in a study that was conducted in Ilorin, North Central, Nigeria. It is also consistent with the results of Fernando et al.[40],who observed that the majority of the HIV-infected patients had Stage 1 category of CKD, hence a finding of predominantly normal GFR in their study population. It may be plausible to state that the subjects with microalbuminuria in this study could have Stage 1 chronic kidney disease, which is commonly not reflective of a low glomerular filtration rate. Similar to non-HIV individuals, the initial stages of HIV-associated CKD are not usually symptomatic, and this may manifest as a normal or minimal reduction in glomerular filtration rate.
There was also no remarkable difference in the estimated glomerular filtration rate (e-GFR) among subjects receiving nucleotide reverse transcriptase inhibitors (NRTI) such as tenofovir disoproxil fumarate, or protease inhibitor (PI) such as lopinavir relative to the group that was not treated with either category of drug. This is similar to the findings by Pontrelli et al.[55] It is also consistent with studies on ART-treated children on TDF-containing regimens, which found no evidence of impaired glomerular filtration rate.[56,57] Similarly, no significant difference existed between the urine albumin–creatinine ratio and the classes of drugs in the study.
None of the HIV-positive subjects in the study had advanced clinical stages of HIV disease (all of them had either Stage I or II of the disease) at the time of clinical assessment in all age groups. This may partly be due to the effectiveness of HAART, which is contributory to the predominantly normal CD-4 cell counts, normal cystatin C level and its e-GFRcys. Meanwhile, as reported by Esezobor et al.[20] in Lagos, serum cystatin C level, its e-GFRcys and proportions of children with abnormally high serum cystatin C levels were not significantly different between the HIV-infected children with advanced disease and those with milder disease.
The relatively small sample size of the study could have underpowered the ability to detect any substantial difference in serum cystatin C and its e-GFR. However, the use of urine albumin–creatinine ratio instead of dipstick for evaluation of proteinuria is a major strength of the study. The urine albumin–creatinine ratio eliminates the dilutional effect of urine on the detection of albuminuria or proteinuria by the common dipstick method. This is especially critical in children, who may need to drink fluids before collecting urine in an ambulatory setting. Also, urine protein detection by fluorescence immunoassay instead of dipstick conferred a high sensitivity for the detection of glomerular proteinuria in the study.
Conclusion
This study showed that albuminuria was high among HIV-positive children on HAART, despite their normal serum levels of cystatin C and its estimated GFR. The prolonged duration of HIV/AIDS was associated with the occurrence of microalbuminuria, which could be an early indicator of subclinical kidney impairment among HIV-infected subjects. The findings of this study indicate a high risk of cardiorenal complications among HIV-infected children, notwithstanding the utilization of HAART. Consequently, regular renal and cardiovascular assessments are warranted for this vulnerable population to mitigate potential morbidity and mortality. However, further research is necessary to elucidate the pathogenesis, progression, and long-term outcomes of individuals with this finding.
Acknowledgments
The authors acknowledge and appreciate the staffs of the Department of Chemical Pathology for their assistance during the sample analyses.
Ethical approval
The research/study approved by the Institutional Review Board at Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, number ERC/2021/06/14, dated 14th June 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- UNAIDS. Global AIDS Update 2022. Available: https://www.unaids.org/en [Last accessed 2024 Jul 20]
- Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195-202.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the management of chronic kidney disease in HIV-infected patients: Recommendations of the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2005;40:1559-85.
- [CrossRef] [PubMed] [Google Scholar]
- HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis. 2006;42:1488-95.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of impaired renal function among HIV infected patients commencing highly active antiretroviral therapy in Jos, Nigeria. Niger Med J. 2011;52:182-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and pattern of human immunodeficiency virus-associated nephropathy among human immunodeficiency virus-positive children at the University of Maiduguri Teaching Hospital, Nigeria. Saudi J Kidney Dis Transpl. 2019;30:843-852.
- [CrossRef] [PubMed] [Google Scholar]
- Renal impairment in a rural African antiretroviral programme. BMC Infect Dis. 2009;9:143.
- [CrossRef] [PubMed] [Google Scholar]
- Renal disease in an antiretroviral-naïve HIV-infected outpatient population in Western Kenya. Nephrol Dial Transplant. 2007;22:2208-12.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic kidney disease in newly diagnosed patients with Human immunodeficiency virus in Ilorin, Nigeria. J Bras Nefrol. 2015;37:177-84.
- [CrossRef] [PubMed] [Google Scholar]
- Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59:653-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of serum cystatin C, serum creatinine, measured GFR, and estimated GFR to assess the risk of kidney failure in American Indians with diabetic nephropathy. Am J Kidney Dis. 2013;62:33-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Simple cystatin C formula compared to sophisticated CKD-EPI formulas for estimation of glomerular filtration rate in the elderly. Ther Apher Dial. 2011;15:261-8.
- [CrossRef] [PubMed] [Google Scholar]
- Simple cystatin C formula for estimation of glomerular filtration rate in overweight patients with diabetes mellitus type 2 and chronic kidney disease. Exp Diabetes Res. 2012;2012:179849.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Persistent microalbuminuria in human immunodeficiency virus infected children in kano, Nigeria. Int J Nephrol. 2014;2014:567838.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of tenofovir disoproxil fumarate on risk of renal abnormality in HIV-1-infected children on antiretroviral therapy: A nested case-control study. AIDS. 2010;24:525-34.
- [CrossRef] [PubMed] [Google Scholar]
- Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49-61.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney function of HIV-infected children in Lagos, Nigeria: Using Filler’s serum cystatin C-based formula. J Int AIDS Soc. 2010;13:17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cystatin C-based evaluation of kidney function of HIV-infected children in Benin City, Southern Nigeria. Int J Nephrol. 2012;2012:861296.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981-5.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal assessment of metabolic abnormalities in adolescents and young adults with HIV-infection acquired perinatally or in early childhood. Metabolism. 2011;60:874-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal function abnormalities in HIV-infected children and adolescents on antiretroviral therapy at the University of Abuja Teaching Hospital, Gwagwalada, Nigeria: A Cross-sectional study. IJTDH. 2017;26:1-12.
- [CrossRef] [Google Scholar]
- Renal abnormalities among HIV infected children at Muhimbili National Hospital (MNH)-Dar es Salaam, Tanzania. BMC Nephrol. 2016;17:30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Relevance of microalbuminuria in screening for HIV-associated nephropathy. http://hdl.handle.net/10539/7650. [Last accessed 2021 June 30].
- [Google Scholar]
- Prevalence of persistent microalbuminuria and associated factors among HIV infected children attending a Tertiary Hospital in Northern Tanzania: A cross sectional, analytical study. Pan Afr Med J. 2015;20:251.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Microalbuminuria in a cohort of ambulatory HIV-positive Nigerians. J Nephrol Ther. 2014;4:179.
- [CrossRef] [Google Scholar]
- Screening for microalbuminuria in HIV-Positive children in Enugu. Int J Nephrol. 2012;2012:805834.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Microalbuminuria in children with human immunodeficiency virus (HIV) infection in Port Harcourt, Nigeria. Niger J Med. 2010;19:298-301.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of proteinuria among HIV-infected children attending a tertiary hospital in Lagos, Nigeria. J Trop Pediatr. 2010;56:187-90.
- [CrossRef] [PubMed] [Google Scholar]
- Micro-albuminuria in adolescent/young adult offsprings of hypertensive Nigerian adults-A preliminary report. Niger J Clin Pract.. 2004;7(2):60-64.
- [Google Scholar]
- Prevalence of microalbuminuria among secondary school children. Afr Health Sci. 2012;12:140-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genetics of common progressive renal disease. Kidney Int Suppl 2005:S41-5.
- [CrossRef] [Google Scholar]
- Genetic factors in end-stage renal disease. Kidney Int Suppl 2005:S46-9.
- [CrossRef] [Google Scholar]
- Genetic susceptibility to HIV-associated nephropathy. J Clin Invest. 2009;119:1085-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HIV infection and kidney disease: Literature Review. Infect Dis Trop Med. 2015;1:e195.
- [Google Scholar]
- Persistent proteinuria as an indicator of renal disease in HIV-infected children. Paediatr Indones. 2016;56:343-9.
- [Google Scholar]
- Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci. 2008;335:89-94.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of renal disease in Nigerian children infected with the human immunodeficiency virus and on highly active anti-retroviral therapy. Saudi J Kidney Dis Transpl. 2013;24:172-7.
- [CrossRef] [PubMed] [Google Scholar]
- Renal function tests: A clinical laboratory perspective. Lab Med. 2007;38:295-304.
- [CrossRef] [Google Scholar]
- Kidney function tests. In: Textbook of biochemistry for Medical students. 2017. p. :370-83.
- [Google Scholar]
- [HIV-1 and renal cells: Pathogenesis of HIV-associated nephropathy] G Ital Nefrol. 2005;22:569-80.
- [PubMed] [Google Scholar]
- HIV-associated nephropathy is a late, not early, manifestation of HIV-1 infection. Kidney Int. 1999;55:1036-40.
- [CrossRef] [PubMed] [Google Scholar]
- Tenofovir-induced nephrotoxicity: A retrospective cohort study. Med J Malaysia. 2016;71:308-312.
- [PubMed] [Google Scholar]
- Tenofovir-associated kidney disease in Africans: A systematic review. AIDS Res Ther. 2019;16:12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Determinants of risk factors for renal impairment among HIV-infected patients treated with tenofovir disoproxil fumarate-based antiretroviral regimen in Southern Vietnam. Biomed Res Int. 2020;2020:7650104.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- [Prevalence of proteinuria and its association with HIV/AIDS in Congolese children living in Kinshasa, Democratic Republic of Congo] Nephrol Ther. 2012;8:163-7.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22:863-7.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in antiretroviral therapy use and survival rates for a large cohort of HIV-infected children and adolescents in the United States, 1989-2001. J Acquir Immune Defic Syndr. 2005;38:488-94.
- [CrossRef] [PubMed] [Google Scholar]
- Nephrotoxicity of HAART. AIDS Res Treat. 2011;2011:562790.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cystatin C and creatinine in an HIV cohort: The nutrition for healthy living study. Am J Kidney Dis. 2008;51:914-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-45.
- [CrossRef] [PubMed] [Google Scholar]
- Renal function in HIV-infected children and adolescents treated with tenofovir disoproxil fumarate and protease inhibitors. BMC Infect Dis. 2012;12:18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics. 2005;116:e846-54.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term renal safety of tenofovir disoproxil fumarate in vertically HIV-infected children, adolescents and young adults: A 60-month follow-up study. Clin Drug Investig. 2011;31:407-15.
- [CrossRef] [PubMed] [Google Scholar]






